From Silent Spring to Sustainable Labs: The Historical Context and Modern Applications of Green Chemistry in Drug Development
This article traces the evolution of the sustainable chemistry movement from its foundational environmental protests to its current status as a driver of innovation in pharmaceutical research and development.
From Silent Spring to Sustainable Labs: The Historical Context and Modern Applications of Green Chemistry in Drug Development
Abstract
This article traces the evolution of the sustainable chemistry movement from its foundational environmental protests to its current status as a driver of innovation in pharmaceutical research and development. It explores the historical catalysts, from Rachel Carson's 'Silent Spring' to the formalization of the Twelve Principles, that shaped green chemistry. For researchers and drug development professionals, the content provides a methodological guide to applying sustainable practices, including solvent-free synthesis and AI-driven reaction optimization. It further addresses troubleshooting common implementation challenges and validates the approach through case studies of award-winning industrial applications and emerging trends poised to redefine sustainable biomedical research.
The Roots of a Revolution: Tracing the Environmental Catalysts that Forged Green Chemistry
The period preceding the 1990s established a foundational environmental paradigm characterized by a reactive approach to pollution. This framework, known as end-of-pipe treatment, focused on containing or treating waste streams after their generation, rather than preventing pollution at its source [1]. The model emerged alongside a growing public consciousness about environmental degradation, fueled by visible ecological crises and seminal scientific writings that collectively spurred legislative action and formed the early environmental movement [2]. This whitepaper examines the technological, regulatory, and social drivers of this paradigm, providing researchers and drug development professionals with a historical context for the subsequent shift towards sustainable chemistry and green manufacturing principles.
Historical Context: The Rise of Environmental Awareness
The end-of-pipe approach was not an isolated technological strategy but a response to a specific historical context marked by escalating pollution and growing public demand for action.
- Early Conservation (Late 19th - Early 20th Century): The initial movement focused on sustainable resource management and preserving wilderness areas. Key milestones included the establishment of the first national parks like Yellowstone (1872) and conservation groups like the Sierra Club (1892) [2]. This era was guided by a conservation ethic, exemplified by Theodore Roosevelt's expansion of national forests and parks [3].
- The Modern Environmental Movement (1960s-1970s): A pivotal shift occurred in the 1960s as concern broadened from conservation to pervasive air and water pollution [3]. Rachel Carson's Silent Spring (1962) warned of pesticide devastation, becoming a bestseller with immense global impact [2]. Highly visible environmental disasters cemented public consciousness:
- Legislative and Institutional Response: Public outcry translated into unprecedented policy action. The U.S. established the Environmental Protection Agency (EPA) in 1970, and the first Earth Day drew 20 million participants [2]. Landmark legislation, including the Clean Air Act (1963, expanded 1970), the Clean Water Act (1972), and the Endangered Species Act (1973), created a new regulatory framework mandating pollution control [2]. These laws primarily set limits for pollutant concentrations in emissions and effluent, making end-of-pipe technologies the most direct compliance pathway [1].
Defining the End-of-Pipe Approach
End-of-pipe solutions represent a class of environmental management strategies focused on treating pollutants or waste streams after they have been generated by a process or activity, immediately before release into the environment [1]. The core principle is interception and remediation at the point of discharge. This fundamentally differs from preventative measures that aim to stop pollution at its source.
- Core Philosophy: The approach is inherently reactive. It manages the symptoms of pollution (the waste stream) rather than addressing the root cause within the industrial process itself [1].
- Objective: The primary goal is to reduce the pollutant load to levels deemed acceptable by regulatory standards before release occurs, thereby mitigating immediate environmental harm [1].
- Regulatory Driver: Widespread adoption was largely driven by early environmental regulations that set concentration limits for discharges. This framework offered industries a clear, measurable target: install treatment technology to clean the waste stream before release [1].
Key End-of-Pipe Technologies and Methodologies
The following table catalogs major end-of-pipe technologies, their mechanisms, and typical applications, providing a reference for the technical solutions of the era.
Table 1: Key End-of-Pipe Technologies and Their Applications
| Pollutant Type | Medium | Technology | Mechanism of Action | Typical Application |
|---|---|---|---|---|
| Particulate Matter | Air | Electrostatic Precipitator (ESP) | Uses an electrostatic charge to attract and remove particles from a flowing gas [1]. | Power plants, heavy industries [1]. |
| SO₂ & Gaseous Pollutants | Air | Scrubbers (e.g., Flue-Gas Desulfurization) | Removes gaseous pollutants via contact with a liquid or dry sorbent, neutralizing acids [1]. | Smelters, chemical plants, power generation [1]. |
| Organic Waste (BOD/COD) | Water | Biological Treatment (e.g., Activated Sludge) | Uses microorganisms to biologically degrade organic pollutants in wastewater [1]. | Municipal and industrial wastewater treatment plants [1]. |
| Heavy Metals | Water | Chemical Precipitation | Adds chemicals to wastewater to convert dissolved metals into insoluble solid particles for removal [1]. | Electroplating, metal finishing, mining effluent [1]. |
| Automotive Emissions | Air | Catalytic Converter | Converts toxic combustion byproducts (CO, NOx, hydrocarbons) into less harmful substances via catalytic reaction [1]. | Automotive exhaust systems [1]. |
| Landfill Gas (Methane) | Waste | Landfill Gas Collection System | Captures methane and other gases produced by decomposing waste via wells and piping [1]. | Municipal solid waste landfills [1]. |
Experimental and Operational Protocols
Implementing these technologies required standardized methodologies to ensure compliance and operational efficacy. Key procedural steps included:
- Source Characterization: Initial waste stream analysis to determine pollutant concentration, flow rate, temperature, and chemical composition. This informed the selection and sizing of the treatment technology [1].
- Technology-Specific Workflows:
- For Scrubbers: The protocol involved the continuous injection of the exhaust stream into a reaction vessel, simultaneous introduction of sorbent slurry (e.g., limestone), and subsequent collection of reaction byproducts (e.g., gypsum sludge) for disposal [1].
- For Wastewater Treatment: A multi-stage process was employed: Primary (physical screening and sedimentation), Secondary (biological degradation in aeration basins), and Tertiary (chemical or filtration polishing to remove specific contaminants like phosphorus or metals) [1].
- Performance Monitoring: Continuous or periodic sampling and analysis of treated effluent or emissions to verify compliance with regulatory discharge permits. This often involved measuring pH, suspended solids, and specific chemical concentrations [1].
The "Scientist's Toolkit": Research and Reagent Solutions for Environmental Analysis
The development and monitoring of end-of-pipe technologies relied on a suite of analytical methods and reagents. This toolkit was essential for quantifying pollution and verifying treatment efficacy.
Table 2: Essential Analytical Reagents and Methods for Pollution Monitoring
| Reagent/Method | Primary Function | Application in End-of-Pipe Analysis |
|---|---|---|
| Nessler's Reagent | Colorimetric detection of ammonia. | Measuring ammonia nitrogen levels in wastewater treatment effluent to assess biological process health [1]. |
| Chemical Oxygen Demand (COD) Test | Quantifies organic pollutants. | Evaluating the oxygen-demanding strength of industrial and municipal wastewaters pre- and post-treatment [1]. |
| Atomic Absorption (AA) Spectroscopy | Detection of metal elements. | Measuring concentrations of heavy metals (e.g., Pb, Cd, Hg) in wastewater and sludge to ensure regulatory compliance [1]. |
| High-Volume Air Sampler | Particulate matter collection. | Gravimetric analysis of total suspended particulates (TSP) and PM₁₀ in industrial air emissions [1]. |
| pH Indicators & Buffers | Measure and control acidity/alkalinity. | Critical for optimizing chemical precipitation processes and monitoring final effluent pH before discharge [1]. |
Critical Analysis: Limitations and the Path to a New Paradigm
While effective for compliance, the end-of-pipe paradigm contained critical flaws that ultimately spurred the development of more sustainable approaches.
Inherent Limitations:
- Resource Inefficiency: The approach does not fundamentally alter the processes that generate pollution, thereby perpetuating resource waste and inefficiency [1].
- Secondary Waste Streams: Treatment processes often create new waste challenges, such as scrubber sludge or spent solvents, which require their own disposal protocols and containment [1].
- High Operational Costs: These systems incur significant ongoing costs for energy, chemical sorbents, and maintenance, leading to a continuous financial drain [4].
- Strategic Position in the Pollution Hierarchy: The universally accepted pollution hierarchy ranks environmental strategies in order of desirability: Prevention is highest, followed by Minimization, Reuse/Recycling, Treatment, and finally Disposal. End-of-pipe solutions occupy the "Treatment" level, signifying they are a less desirable, reactive measure [1].
The Regulatory and Economic Shift: The Pollution Prevention Act of 1990 marked a formal U.S. policy shift, declaring that "pollution should be prevented or reduced at the source whenever feasible" [5]. This policy change began to alter the economic calculus, encouraging source reduction over waste treatment.
The following diagram illustrates the conceptual and operational differences between the end-of-pipe paradigm and the emerging pollution prevention framework that would gain prominence in the 1990s.
Diagram Title: Reactive vs. Proactive Environmental Management
The pre-1990s paradigm of end-of-pipe treatment was an essential, albeit transitional, phase in environmental protection. It successfully mitigated the most visible and acute forms of pollution through technological innovation driven by regulatory pressure and public advocacy [2] [1]. However, its reactive nature, operational costs, and creation of secondary wastes revealed its systemic limitations [4] [1]. This framework's position within the pollution hierarchy—below prevention and minimization—highlighted its role as a tactical, not strategic, solution. The experiences and shortcomings of this era were instrumental in paving the way for the principles of green chemistry and sustainable engineering, which seek to prevent waste at the molecular level and design inherently safer, more efficient processes [5]. For researchers today, understanding this evolution is critical for appreciating the foundational logic behind modern sustainable science.
The modern sustainable chemistry movement did not emerge in a vacuum; it was catalyzed by a series of pivotal environmental wake-up calls that exposed the profound consequences of chemical pollution on human health and ecological systems. Three landmark events—the 1962 publication of Rachel Carson's Silent Spring, the 1970s Love Canal toxic waste crisis, and the passage of the 1990 Pollution Prevention Act—collectively shifted scientific, regulatory, and public paradigms from pollution control to pollution prevention. This whitepaper examines these critical milestones within the broader historical context of sustainable chemistry research, tracing their role in transforming chemical design, manufacturing, and regulatory frameworks. For researchers and drug development professionals, understanding this evolution is essential for advancing greener synthetic pathways, reducing hazardous waste generation, and embracing the principles of green chemistry that now underpin cutting-edge sustainable research.
Silent Spring: The Catalyst for Environmental Consciousness
Historical Context and Scientific Foundation
Published in 1962, Rachel Carson's Silent Spring represented a paradigm shift in scientific and public understanding of pollution's interconnected impacts. Carson, a marine biologist with the U.S. Fish and Wildlife Service from 1936 to 1952, synthesized scientific evidence on the ecological harm caused by synthetic pesticides, particularly DDT (dichloro-diphenyl-trichloroethane) [6]. Her work emerged during a post-WWII era when science and industry were enthusiastically translating wartime technologies into commercial products, with U.S. production of DDT leaping from 4,366 tons in 1944 to a peak of 81,154 tons in 1963 [6]. Carson documented how pesticides not only targeted pests but also traveled through ecosystems, accumulated in food chains, harmed wildlife, and posed potential human health risks, including carcinogenesis [6] [7].
Carson's methodological approach was notable for its interdisciplinary rigor, citing dozens of scientific reports, conducting interviews with leading experts, and reviewing materials across disciplines [6]. She compiled evidence on chemical impacts across aerial sprayings, industrial settings, and food applications, characterizing these impacts in ecological terms rather than simply assessing chemical efficacy [6]. This systems-thinking approach revealed the interconnectedness of biological systems—a foundational concept for modern green chemistry.
Key Findings and Impact
Silent Spring introduced several revolutionary concepts to the public consciousness: that spraying chemicals to control insect populations could kill birds that feed on dead or dying insects; that chemicals travel through environments and food chains; that persistent chemicals could accumulate in fat tissues causing medical problems later; and that chemicals could be transferred generationally from mothers to their young [6]. Importantly, Carson did not advocate for an outright ban on pesticides but rather for caution, further study, and development of biological alternatives [6] [7].
The book sparked immediate controversy, drawing fierce opposition from chemical companies but ultimately resonating with political leaders and the public [6] [8]. The legacy of Silent Spring includes direct policy impacts such as the ban on domestic DDT use in 1972 due to its widespread overuse and harmful environmental impact, the establishment of the U.S. Environmental Protection Agency in 1970, and the passage of numerous environmental laws [6] [9]. The book also promoted a paradigm shift in how chemists practice their discipline, helping establish a new role for chemists in investigating the impact of human activity on the environment [6].
Table 1: Key Environmental Legislation Following Silent Spring
| Legislation | Year | Key Provisions | Impact on Chemical Industry |
|---|---|---|---|
| National Environmental Policy Act | 1969 | Established national environmental policy and created Council on Environmental Quality | Required environmental impact statements for major projects [9] |
| Clean Air Act | 1970 | Regulated air emissions from stationary and mobile sources | Set limits on hazardous air pollutants from chemical plants [10] |
| Clean Water Act | 1974 | Established wastewater standards for industry | Controlled chemical discharges into water systems [10] |
| Toxic Substances Control Act | 1976 | Gave EPA authority to require reporting and restrictions on chemical substances | Regulated new and existing chemicals in commerce [10] |
Love Canal: The Consequences of Improper Chemical Disposal
Background and Discovery
The Love Canal tragedy represents one of the most appalling environmental disasters in American history, directly demonstrating the human health consequences of improper chemical waste management [11]. From 1942 to 1952, Hooker Chemical Company dumped approximately 19,800 metric tonnes of chemical byproducts from manufacturing dyes, perfumes, and solvents for rubber and synthetic resins into the abandoned Love Canal in Niagara Falls, New York [12]. The canal was subsequently covered with clay and sold to the local school district for $1 in 1953, with a deed containing a liability limitation clause attempting to release Hooker from future legal obligations [12].
By the late 1970s, following record rainfall, the disaster emerged as corroding waste-disposal drums broke through the ground in residents' backyards, with chemical puddles forming in yards and basements, and noxious substances contaminating the air [11]. The New York State Health Department investigated disturbingly high rates of miscarriages and birth defects in the area, while residents showed high white-blood-cell counts, a possible precursor to leukemia [11]. One resident recounted two grandchildren with birth defects—one born deaf with a cleft palate and another with an eye defect—highlighting the human tragedy [11].
Methodological Approaches in Environmental Assessment
The investigation of Love Canal employed multiple scientific methodologies to document contamination and health impacts:
- Environmental Sampling: Testing of soil, groundwater, and air for chemical contaminants, identifying 82 different compounds, including 11 suspected carcinogens [11]
- Health Epidemiology Studies: Investigation of miscarriage rates, birth defects, and potential leukemia cases through community health assessment [11]
- Engineering Assessments: Evaluation of drum integrity, leaching mechanisms, and containment failure analysis [11]
These methodologies established critical cause-effect relationships between chemical exposure and human health impacts, providing a template for future hazardous waste site investigations.
Policy and Regulatory Outcomes
Love Canal had profound regulatory consequences, most notably prompting the passage of the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) of 1980, commonly known as Superfund [12]. This law:
- Established a federal "Superfund" to clean up uncontrolled hazardous waste sites
- Created a system for determining responsible parties for contamination
- Enabled both short-term removals and long-term remedial actions
- Implemented a National Priorities List for prioritizing cleanup sites
The Love Canal site itself was proposed for the Superfund National Priorities List on December 30, 1983, formally listed on September 8, 1984, construction was completed on September 29, 1998, and it was officially deleted from the list on September 30, 2004, after 21 years of cleanup [12].
Table 2: Love Canal Timeline and Impacts
| Year | Event | Significance |
|---|---|---|
| 1942-1952 | Hooker Chemical uses Love Canal as dump site | 19,800 metric tonnes of chemical waste buried [12] |
| 1953 | Hooker sells property to school board for $1 | Deed includes liability limitation clause [12] |
| 1950s | Homes and school built on and near canal | Approximately 100 homes and school exposed [12] |
| 1977 | Contamination discovered | 82 compounds identified, 11 suspected carcinogens [11] |
| 1978 | Emergency declarations | First emergency funds for non-natural disaster [11] |
| 1980 | CERCLA (Superfund) passed | Direct response to Love Canal and similar sites [12] |
Pollution Prevention Act of 1990: Codifying the Paradigm Shift
Legislative Framework and Definitions
The Pollution Prevention Act (PPA) of 1990 marked a fundamental shift in U.S. environmental policy, establishing a national policy that pollution should be prevented or reduced at the source whenever feasible [13] [14]. The legislation declared a hierarchical approach to environmental management: first, prevent or reduce pollution at the source; second, recycle in an environmentally safe manner; third, treat pollution; and finally, dispose or release into the environment only as a last resort [13].
The PPA defined "source reduction" as any practice that reduces the amount of any hazardous substance, pollutant, or contaminant entering any waste stream or otherwise released into the environment prior to recycling, treatment, or disposal [13]. This specifically included:
- Equipment or technology modifications
- Process or procedure modifications
- Reformulation or redesign of products
- Substitution of raw materials
- Improvements in housekeeping, maintenance, training, or inventory control
Crucially, the Act explicitly excluded practices that alter the physical, chemical, or biological characteristics or volume of hazardous substances through processes not integral to production [13].
Implementation Mechanisms
The PPA established several key implementation mechanisms:
- EPA Office Establishment: Required the EPA Administrator to establish an independent office to carry out PPA functions and develop a strategy to promote source reduction [13]
- State Technical Assistance Grants: Created matching grant programs to states for promoting source reduction techniques by businesses [13]
- Source Reduction Clearinghouse: Established a clearinghouse to compile information including a computer database on management, technical, and operational approaches to source reduction [13]
- Toxic Chemical Reporting: Expanded the Toxics Release Inventory (TRI) to require facilities to report on source reduction and recycling activities [13] [14]
These mechanisms collectively shifted the regulatory focus from end-of-pipe treatment to preventative approaches, encouraging innovation in chemical processes and products.
Evolution of Green Chemistry Principles and Practices
Historical Development
The environmental awareness raised by Silent Spring and Love Canal, combined with the policy framework of the PPA, created fertile ground for the emergence of green chemistry as a distinct scientific field. In the 1990s, this evolution accelerated with several key developments:
- 1991: The term "Green Chemistry" was coined by staff of the EPA Office of Pollution Prevention and Toxins [9]
- 1994: The first symposium "Benign by Design: Alternative Synthetic Design for Pollution Prevention" was held in Chicago [9]
- 1995: Establishment of the annual Presidential Green Chemistry Challenge Awards [9]
- 1997: Creation of the Green Chemistry Institute (GCI) as an independent nonprofit [10]
- 1998: Paul Anastas and John C. Warner co-authored Green Chemistry: Theory and Practice, outlining the 12 Principles of Green Chemistry [9] [10]
The field gained scientific credibility through Nobel Prizes in 2001 (Knowles, Noyori, Sharpless for chiral catalysis) and 2005 (Chauvin, Grubbs, Schrock for metathesis reactions), both recognizing research areas aligned with green chemistry principles [9].
The Scientist's Toolkit: Green Chemistry Research Reagents
Table 3: Research Reagents and Alternatives in Green Chemistry
| Reagent/Category | Traditional Examples | Green Alternatives | Function & Applications |
|---|---|---|---|
| Solvents | Halogenated (CH₂Cl₂, CHCl₃), BTEX solvents | Supercritical CO₂, water, ionic liquids, bio-based solvents | Reaction media with reduced toxicity and environmental impact [9] |
| Catalysts | Heavy metals (Pd, Pt) | Biocatalysts, organocatalysts, immobilized catalysts | Increase efficiency, reduce energy requirements, enable alternative pathways [10] |
| Oxidizing Agents | Chromium(VI) reagents, peracids | Hydrogen peroxide, oxygen (air), enzymatic oxidation | Safer stoichiometric oxidants with less hazardous byproducts [15] |
| Reducing Agents | Metal hydrides (LiAlH₄) | Catalytic hydrogenation, biomimetic reductants | Safer reduction processes with better atom economy [15] |
| Feedstocks | Petroleum-based | Biomass-derived, renewable feedstocks | Sustainable carbon sources with reduced lifecycle impacts [9] |
Analytical Methodologies and Experimental Protocols
Environmental Monitoring Techniques
The wake-up calls of Silent Spring and Love Canal drove innovations in environmental monitoring methodologies that remain essential today:
- Chromatographic Methods: Advanced GC-MS and LC-MS protocols for detecting pesticide residues and chemical contaminants at parts-per-billion levels in environmental and biological samples
- Ecological Impact Assessment: Standardized protocols for measuring bioaccumulation factors (BAFs) and biomagnification in food webs
- Toxicological Screening: Cell-based assays and animal models for assessing chronic toxicity, endocrine disruption, and carcinogenicity of chemicals
- Environmental Fate Studies: Radiolabeled compound tracking to determine persistence, degradation pathways, and metabolite formation
Green Chemistry Metrics and Assessment
Green chemistry developed standardized metrics to evaluate the environmental performance of chemical processes:
- Atom Economy: Calculation of the proportion of reactant atoms incorporated into the final product
- Environmental Factor (E-Factor): Total waste produced per unit of product (kg waste/kg product)
- Process Mass Intensity (PMI): Total mass used in a process per unit of product (kg total materials/kg product)
- Life Cycle Assessment (LCA): Holistic evaluation of environmental impacts across a product's entire lifecycle
Conceptual Framework and Signaling Pathways
The evolution of sustainable chemistry represents a fundamental paradigm shift in how chemical processes are conceived, designed, and implemented. The diagram below illustrates this conceptual framework and the relationships between key historical events, regulatory responses, and scientific developments.
The trajectory from Silent Spring to Love Canal to the Pollution Prevention Act represents a critical evolution in environmental thought—from recognizing problems to mandating preventative solutions. For today's researchers and drug development professionals, this historical context provides both a moral imperative and practical framework for advancing sustainable chemistry. The current challenges of climate change, resource depletion, and continuing chemical pollution demand renewed commitment to green chemistry principles. Future directions include advancing biocatalysis, continuous flow chemistry, artificial intelligence-guided molecular design, and the transition from petroleum to renewable feedstocks. By building upon the legacy of these landmark wake-up calls, the scientific community can continue transforming chemical practice to harmonize human well-being with planetary health.
The formalization of green chemistry as a distinct scientific discipline originated within the U.S. Environmental Protection Agency (EPA) in the early 1990s. This transformative approach emerged as a strategic response to the Pollution Prevention Act of 1990, which marked a fundamental policy shift from pollution control to pollution prevention. The EPA's Office of Pollution Prevention and Toxics (OPPT) was instrumental in catalyzing this movement, seeding initial research grants and building a foundational framework that translated policy into a new chemical design paradigm. This whitepaper details the historical context, key actors, and foundational programs established by OPPT that propelled green chemistry from a conceptual idea into a global sustainability framework essential for modern researchers and drug development professionals.
The period preceding the 1990s was characterized by a "command and control" or "end-of-pipe" regulatory approach to environmental management, focusing on treating and disposing of hazardous waste after it was created [16]. This began to change with growing environmental consciousness throughout the 1960s and 1970s, catalyzed by events such as the Cuyahoga River fire and the publication of Rachel Carson's Silent Spring, which ultimately led to the establishment of the EPA in 1970 [17]. The critical turning point for green chemistry, however, was the Pollution Prevention Act of 1990, which established a new U.S. national policy: pollution should be prevented or reduced at the source whenever feasible [16]. This legislation championed cost-effective changes in products, processes, and the use of raw materials over recycling, treatment, and disposal [16].
It was within this policy context that the EPA's OPPT moved away from a purely regulatory role. The office began championing a proactive approach, seeking to redesign chemical products and processes before they posed a risk to human health or the environment [16]. This philosophical and strategic pivot laid the essential groundwork for the birth of green chemistry as a formal field of study and practice.
The OPPT's Foundational Role in Establishing Green Chemistry
Key Milestones and Actions
The Office of Pollution Prevention and Toxics acted as the central engine for initializing the green chemistry movement through two primary, interconnected mechanisms: research funding and programmatic development.
Research Grant Program (1991): In direct response to the Pollution Prevention Act, OPPT launched a seminal research grant program in 1991, then termed "Alternative Synthetic Routes for Pollution Prevention" [16] [18]. This program provided crucial early funding to redesign existing chemical products and processes to reduce their impacts, representing the first major governmental investment in the concepts that would become green chemistry.
Program Expansion and Renaming (1992): Within a year, the program's scope expanded to include other topics like environmentally friendly solvents and safer chemical compounds. It was at this point that the initiative officially adopted the name "green chemistry," solidifying a new identity for this emerging field [18].
Partnership with the National Science Foundation (NSF): The EPA, through OPPT, partnered with the NSF in the early 1990s to fund basic research in green chemistry, lending further scientific credibility and academic reach to the nascent field [16].
The following timeline visualizes the key initiatives led by the OPPT and their pivotal role in the early development of green chemistry.
From Concept to Formal Principles: The Presidential Green Chemistry Challenge
A cornerstone of OPPT's strategy to advance green chemistry was the creation of the Presidential Green Chemistry Challenge Awards in 1996 [16]. These awards were designed to recognize and promote real-world academic and industrial technologies that incorporated green chemistry, effectively creating a repository of success stories [16]. This program served a critical function in moving the field from theoretical discourse to demonstrated application, providing tangible case studies for educational and research purposes.
The intellectual framework of the field was codified in 1998 with the publication of the 12 Principles of Green Chemistry by Paul Anastas and John Warner [16] [18]. These principles provided a clear, comprehensive set of design guidelines, encompassing concepts such as waste prevention, atom economy, safer solvents and auxiliaries, and design for degradation [16]. This was a pivotal moment that gave the global research community a shared vocabulary and a systematic approach for designing safer chemical products and processes.
Quantitative Tracking and Industry Adoption
The EPA developed concrete mechanisms to track the adoption of green chemistry practices in industry, primarily through the Toxics Release Inventory (TRI) Program. The TRI tracks industrial implementation using specific source reduction codes, creating a valuable dataset for analyzing trends [19].
Table 1: TRI Green Chemistry and Engineering Tracking Codes
| Code | Practice | Primary Focus |
|---|---|---|
| S01 | Substituted a fuel | Material Substitution |
| S02 | Substituted an organic solvent | Material Substitution |
| S03 | Substituted raw materials, feedstock, or reactant chemical | Material Substitution |
| S04 | Substituted manufacturing aid, processing aid, or other ancillary chemical | Material Substitution |
| S05 | Modified content, grade, or purity of a chemical input | Material Substitution |
| S11 | Reformulated or developed new product line | Material Substitution |
| S21 | Optimized process conditions to increase efficiency | Process & Equipment Modification |
| S22 | Instituted recirculation within a process | Process & Equipment Modification |
| S23 | Implemented new technology, technique, or process | Process & Equipment Modification |
| S43 | Introduced in-line product quality monitoring or other process analysis system | Process & Equipment Modification |
Source: Adapted from EPA TRI Green Chemistry and Green Engineering Reporting [19]
These codes allow researchers and policymakers to quantitatively monitor the adoption of specific green chemistry strategies, such as solvent substitution (S02) or process optimization (S21), across industrial sectors [19]. The public accessibility of this data via the TRI Toxics Tracker tool makes it a powerful resource for benchmarking and research.
Core Principles and Methodologies for Researchers
The 12 Principles as a Design Framework
For research scientists and drug development professionals, the 12 Principles of Green Chemistry provide a proactive design framework. The central premise, embodied in Principle 1 (Prevention), is that it is inherently safer and more cost-effective to prevent waste than to treat or clean it up after it is formed [16]. This "ounce of prevention" reduces the need for hazard management and minimizes risks from potential accidents or exposures [16].
Key principles highly relevant to pharmaceutical R&D include:
- Principle 3: Less Hazardous Chemical Syntheses: Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
- Principle 5: Safer Solvents and Auxiliaries: The use of auxiliary substances (e.g., solvents, separation agents) should be made unnecessary wherever possible and innocuous when used.
- Principle 9: Catalysis: Catalytic reagents (as selective as possible) are superior to stoichiometric reagents, minimizing energy use and waste by enabling more efficient reactions [19].
Experimental Protocol: A Green Chemistry Workflow for Method Development
Integrating green chemistry into research requires a systematic methodology. The following workflow provides a structured approach for developing chemical syntheses or analytical methods with reduced environmental and health impacts.
This iterative process emphasizes inherent rather than circumstantial safety, ensuring that risk is minimized at the molecular level through design, rather than through added controls or protective equipment [16].
The Research Reagent Toolkit: Essential Materials for Safer Synthesis
A practical application of green chemistry in the laboratory involves substituting hazardous reagents with safer alternatives. The following table details key reagent solutions that align with the principles of green chemistry.
Table 2: Research Reagent Solutions for Safer Chemical Synthesis
| Reagent Category | Function | Traditional Example | Greener Alternative | Principle Addressed |
|---|---|---|---|---|
| Solvents | Substance dissolution, reaction medium | Halogenated (methylene chloride), Benzene | Water, Supercritical CO₂, Ethyl Lactate, Bio-based alcohols [18] | Safer Solvents & Auxiliaries |
| Catalysts | Increase reaction rate/selectivity, regenerated post-use | Stoichiometric reagents (e.g., AlCl₃) | Solid Acid Catalysts, Biocatalysts, Recyclable Metal Complexes [19] | Catalysis, Atom Economy |
| Feedstocks | Starting material for synthesis | Petrochemical derivatives | Biomass-derived sugars, Fatty acids, Agricultural waste streams [16] | Use of Renewable Feedstocks |
| Oxidizing Agents | Selective oxidation reactions | Heavy metal oxidants (CrO₃, KMnO₄) | Hydrogen peroxide (H₂O₂), Molecular Oxygen (O₂) [18] | Less Hazardous Synthesis, Design for Degradation |
Impact and Future Directions in Pharmaceutical and Chemical Research
The adoption of green chemistry has yielded multidimensional impacts, fundamentally shifting research and development in the pharmaceutical and specialty chemical industries. By designing for reduced hazard, companies have lowered the risks of occupational exposure and environmental contamination from accidents or improper disposal [16]. The systems-thinking approach of green chemistry also encourages lifecycle thinking, where the entire lifespan of a chemical product—from feedstock to end-of-life—is considered at the design stage to minimize waste and design for circularity or degradation [19].
Future research, as outlined in EPA's Chemical Safety for Sustainability Strategic Plan, focuses on developing predictive toxicology and advanced tools to make hazard a molecular property as malleable as melting point or color [16] [20]. The next frontier involves treating the 12 Principles not as isolated goals but as a cohesive, mutually reinforcing system to address interconnected sustainability challenges at the molecular level [16].
The genesis of green chemistry is a powerful example of how science policy can catalyze an entire scientific discipline. The EPA's Office of Pollution Prevention and Toxics provided the essential initial catalyst—through funding, program creation, and philosophical leadership—that transformed the mandate of the Pollution Prevention Act into the robust, principled field of green chemistry. For today's researchers and drug development professionals, the OPPT's foundational work provides a proven, effective framework for designing chemical products and processes that align economic viability with environmental responsibility and social good, turning molecular design into a primary strategy for achieving sustainability.
The development of the Twelve Principles of Green Chemistry in the 1990s represented a paradigm shift in how chemists approach the design of chemical products and processes. This formalization occurred against a backdrop of growing environmental awareness that began decades earlier. The 1962 publication of Rachel Carson's "Silent Spring" stimulated the contemporary environmental movement by highlighting the ecological damage caused by pesticides [18] [21]. This was followed by significant milestones including the 1972 Stockholm Conference, which alerted the world to environmental damage from ecosystem depletion, and the 1987 Brundtland Report, which first defined "sustainable development" as meeting present needs without compromising future generations [18].
The U.S. Pollution Prevention Act of 1990 marked a critical turning point by establishing that national policy should eliminate pollution through improved design rather than through treatment and disposal [16] [22]. In response to this legislation, Paul Anastas and John Warner formally articulated the Twelve Principles of Green Chemistry in their 1998 book Green Chemistry: Theory and Practice, providing a systematic framework for designing chemical products and processes that reduce or eliminate the use and generation of hazardous substances [18] [16]. The U.S. Environmental Protection Agency launched its green chemistry program in 1991, and the field gained further recognition with the establishment of the annual Presidential Green Chemistry Challenge Awards in 1996 [16]. This historical trajectory reflects the chemical community's transition from pollution control to pollution prevention, embracing the core philosophy that it is better to prevent waste than to treat or clean it up after it is formed [18] [16].
The Twelve Principles: Framework and Interpretation
Paul Anastas and John Warner's Twelve Principles of Green Chemistry provide a comprehensive design framework for reducing the environmental impact of chemical processes and products across their entire life cycle [23]. These principles have guided academic and industrial innovations for more than two decades, encouraging chemists to pursue inherently safer and more efficient chemical synthesis [23] [16].
Table 1: The Twelve Principles of Green Chemistry with Key Focus Areas
| Principle | Core Concept | Key Focus Areas |
|---|---|---|
| 1. Prevention | Prevent waste rather than treat or clean up | Source reduction, process efficiency [23] |
| 2. Atom Economy | Maximize incorporation of materials into final product | Synthetic route design, molecular efficiency [23] |
| 3. Less Hazardous Chemical Syntheses | Design methods using/generating non-toxic substances | Alternative synthetic pathways, benign reagents [23] |
| 4. Designing Safer Chemicals | Preserve efficacy while reducing toxicity | Structure-activity relationships, toxicology [23] |
| 5. Safer Solvents and Auxiliaries | Minimize auxiliary substance use | Solvent selection, solvent-free reactions [23] |
| 6. Design for Energy Efficiency | Minimize energy requirements of processes | Ambient conditions, process intensification [24] |
| 7. Use Renewable Feedstocks | Utilize biomass rather than depleting resources | Biobased materials, agricultural wastes [24] |
| 8. Reduce Derivatives | Minimize unnecessary functionalization | Protecting group avoidance, direct synthesis [24] |
| 9. Catalysis | Prefer catalytic over stoichiometric reagents | Catalyst design, catalytic cycles [24] |
| 10. Design for Degradation | Design products to break down after use | Biodegradability, environmental persistence [24] |
| 11. Real-time Analysis | Monitor processes to prevent hazardous substance formation | Process analytical technology, in-line monitoring [24] |
| 12. Inherently Safer Chemistry | Choose substances to minimize accident potential | Chemical hazard assessment, process safety [24] |
The principles are interconnected, working together as a cohesive system with mutually reinforcing components rather than as isolated parameters to be optimized separately [16]. The first principle—prevention—is often regarded as the most fundamental, with the other principles representing the "how to" for its achievement [23]. As these principles have been implemented across the chemical enterprise, specific metrics have been developed to quantify their application and effectiveness.
Table 2: Key Green Chemistry Metrics for Process Evaluation
| Metric | Calculation | Interpretation | Ideal Value |
|---|---|---|---|
| E-Factor | Mass of waste ÷ Mass of product [24] | Lower values indicate less waste generation [24] | 0 |
| Atom Economy | (FW of atoms utilized ÷ FW of all reactants) × 100 [24] | Higher % indicates more efficient atom incorporation [23] | 100% |
| Process Mass Intensity (PMI) | Total mass in process ÷ Mass of product [24] | Lower values indicate better material efficiency [23] | 1 |
| EcoScale | 100 - penalty points across multiple categories [24] | Higher scores indicate greener processes [24] | 100 |
Figure 1: Interrelationships Among the Twelve Principles of Green Chemistry
Quantitative Assessment Frameworks in Green Chemistry
The implementation of green chemistry principles requires robust metrics to evaluate and compare the environmental performance of chemical processes. These quantitative tools enable researchers to make data-driven decisions when designing synthetic routes.
Atom Economy: A Fundamental Metric
Atom economy, developed by Barry Trost, evaluates the efficiency of a synthetic method by calculating what percentage of reactant atoms are incorporated into the final desired product versus being wasted as byproducts [23]. This differs from traditional yield calculations, which measure the efficiency of product formation without accounting for wasted starting materials.
For example, in the conversion of 1-butanol to 1-bromobutane:
Even with a 100% yield, the atom economy is only 50%, meaning half the mass of the reactant atoms is wasted in unwanted byproducts [23]. This metric encourages chemists to design syntheses that maximize the incorporation of starting materials into the final product.
Process Mass Intensity and E-Factor
While atom economy focuses on reactants, Process Mass Intensity (PMI) provides a more comprehensive assessment by including all materials used in a process—reactants, solvents, catalysts, and process aids—relative to the mass of product obtained [23] [24]. PMI has become favored in the pharmaceutical industry, where solvents often constitute the bulk of material input [23].
The E-factor, developed by Roger Sheldon, similarly measures environmental impact by calculating the ratio of waste to product mass [24] [22]. Different industry sectors typically operate within characteristic E-factor ranges:
- Oil refining: < 0.1
- Bulk chemicals: 1-5
- Fine chemicals: 5-50
- Pharmaceuticals: 25-100 [24]
These high E-factors in pharmaceutical manufacturing have driven substantial green chemistry innovation in that sector [23].
EcoScale: A Holistic Assessment Tool
The EcoScale provides a multi-criteria evaluation that incorporates yield, cost, safety, technical setup, temperature/time requirements, and workup/purification complexity [24]. It assigns penalty points across these categories, with higher final scores (closer to 100) indicating greener processes. This metric is particularly valuable because it integrates both quantitative and qualitative factors affecting process greenness.
Green Chemistry in Pharmaceutical Research and Development
The pharmaceutical industry has emerged as a significant adopter of green chemistry principles, driven by both environmental concerns and economic imperatives. The high E-factors traditionally associated with drug manufacturing—often exceeding 100 kg waste per kg of active pharmaceutical ingredient (API)—have motivated substantial process improvements [23] [25].
Case Study: Sustainable Drug Discovery at AstraZeneca
AstraZeneca has implemented multiple green chemistry strategies across its drug discovery and development pipeline. These include:
Late-stage functionalization: This technique modifies molecules late in their synthesis, creating "shortcuts" that reduce reaction times and resource-intensive steps. The company has used this approach to generate over 50 different drug-like molecules more sustainably [25]. One notable application enables selective addition of functional groups to drug compounds at precise molecular locations in a single step, dramatically improving synthetic efficiency [25].
Reaction miniaturization: In collaboration with Stockholm University, AstraZeneca has developed approaches using as little as 1mg of starting material to perform thousands of reactions. This high-throughput method allows exploration of a much larger range of drug-like molecules with the same amount of material [25].
Machine learning for reaction optimization: By analyzing large datasets of chemical reactions, machine learning algorithms help predict reaction outcomes and optimize conditions. AstraZeneca has developed models that outperform previous methods for predicting sites of borylation reactions, streamlining development while reducing waste [25].
Sustainable Catalysis in Pharmaceutical Manufacturing
Catalysis represents a cornerstone of green chemistry in pharmaceutical applications, with several innovative approaches being implemented:
Photocatalysis: Visible-light-mediated catalysis enables synthesis of crucial drug building blocks under mild conditions, employing safer reagents and opening new synthetic pathways. AstraZeneca has developed photocatalyzed reactions that remove several stages from cancer drug manufacturing, improving efficiency and reducing waste [25].
Electrocatalysis: This approach uses electricity to drive chemical reactions, offering sustainable routes to organic synthesis while replacing harmful chemical reagents. In one collaborative study, electrocatalysis was applied to selectively attach carbon units to create libraries of drug-like compounds [25].
Biocatalysis: Using enzymes to accelerate chemical reactions often achieves in single steps what requires multiple steps using traditional methods. Advances in computational enzyme design combined with machine learning are expanding the range of available biocatalysts [25].
Sustainable metal catalysis: Replacing precious metals like palladium with more abundant alternatives represents another green chemistry strategy. AstraZeneca has demonstrated that replacing palladium with nickel-based catalysts in borylation reactions reduces CO₂ emissions, freshwater use, and waste generation by more than 75% [25].
Table 3: Research Reagent Solutions for Green Chemistry Applications
| Reagent/Catalyst Type | Function | Green Chemistry Advantages |
|---|---|---|
| Nickel Catalysts | Cross-coupling reactions [25] | Replaces scarce palladium; >75% reduction in CO₂, water use, waste [25] |
| Biocatalysts (Enzymes) | Selective molecular transformations [25] | Single-step processes; renewable; biodegradable [25] |
| Photocatalysts | Light-mediated reactions [25] | Mild conditions; novel reactivities; reduced energy requirements [25] |
| Renewable Solvents | Reaction media [21] | Biobased origins; reduced toxicity; better biodegradability [21] |
| Supported Reagents | Facilitate reactions and separations [24] | Recyclable; reduce waste; improve efficiency [24] |
Figure 2: Green Chemistry Workflow in Pharmaceutical Development
Experimental Protocols and Methodologies
Late-Stage Functionalization Protocol
Late-stage functionalization represents a powerful green chemistry approach that modifies complex molecules at advanced synthetic stages, avoiding the need to reconstruct molecular scaffolds from simpler starting materials [25].
Experimental workflow:
- Substrate preparation: Dissolve the advanced intermediate (typically 0.1-0.5 mmol) in an appropriate green solvent (preferably ethanol, water, or 2-MeTHF).
- Catalyst system selection: Choose from:
- Photoredox catalysts (e.g., Ir(ppy)₃, Ru(bpy)₃²⁺) for radical-mediated functionalization
- Directed C-H activation catalysts (e.g., Pd-based with directing groups)
- Electrocatalytic setups with carbon-based electrodes
- Reaction execution: For photoredox reactions: irradiate with blue LEDs (typically 34W) while stirring at room temperature under nitrogen atmosphere for 2-24 hours.
- Reaction monitoring: Use TLC or UPLC-MS to track reaction progress.
- Product isolation: Employ direct crystallization or chromatography on sustainable supports (such as silica gel from rice husk ash).
- Analysis: Characterize products using NMR, HRMS, and determine purity by HPLC.
Key green chemistry benefits: This methodology typically reduces synthetic steps by 3-5 steps compared to traditional approaches, improving atom economy and reducing PMI by 30-60% [25].
Continuous Flow Photocatalysis Protocol
Continuous flow chemistry represents another green chemistry advancement, particularly when combined with photocatalysis for pharmaceutical applications [25].
Experimental setup:
- Reactor configuration: Use a commercially available or custom-built flow photoreactor with transparent fluoropolymer tubing (e.g., PFA, internal diameter 0.5-1.0 mm) wrapped around a light source.
- Solution preparation: Dissolve substrates (0.1-0.5 M) and photocatalyst (0.5-2 mol%) in degassed solvent mixture.
- Pumping system: Use syringe pumps or peristaltic pumps to maintain precise flow rates (typically 0.1-0.5 mL/min).
- Irradiation: Employ LED arrays at appropriate wavelength (commonly 450 nm for blue light-absorbing catalysts).
- Residence control: Adjust tube length and flow rate to achieve desired residence time (typically 5-30 minutes).
- Product collection: Collect outflow in a receiving flask, often with in-line quenching.
- Workup: Minimal processing required; often direct concentration or crystallization suffices.
Green chemistry advantages: This approach typically demonstrates 20-40% reduction in PMI, 50-80% reduction in reaction time, and improved safety profile compared to batch processes [25].
Current Challenges and Future Directions
Despite significant progress, green chemistry faces several challenges in broader implementation. In developing countries, sustainable chemistry remains a relatively new concept, with university curricula often lacking comprehensive coverage of green chemistry principles [26]. This educational gap creates a barrier to implementing these concepts in regions experiencing growing chemical production [26].
The field is also evolving beyond the original twelve principles to incorporate broader considerations. The emerging concept of "Responsible Research and Innovation" (RRI) seeks to integrate social, ethical, economic, and political dimensions with green chemistry's technical and environmental focus [27]. This approach recognizes that solving sustainability challenges requires interdisciplinary cooperation and systems thinking [28] [27].
Future directions in green chemistry include:
- Integration with One Health approach: This unified perspective recognizes the interconnectedness of human, animal, and environmental health, particularly in developing pharmaceuticals for vector-borne diseases [22].
- Advanced computational tools: Machine learning and artificial intelligence are increasingly being deployed to predict reaction outcomes, optimize conditions, and identify greener synthetic pathways [25] [28].
- Sustainable chemistry metrics harmonization: Efforts are underway to standardize and harmonize metrics across different sectors to facilitate decision-making throughout the value chain [28].
- Interdisciplinary collaboration: Addressing complex sustainability challenges requires collaboration between chemists, toxicologists, process engineers, and social scientists [28].
The 2021 Sustainable Chemistry Research and Development Act in the United States represents significant policy support for these initiatives, mandating the development of a comprehensive federal strategy for advancing sustainable chemistry [28]. As green chemistry continues to evolve, its principles provide a enduring framework for designing chemical products and processes that support both human well-being and environmental sustainability.
The institutionalization of green chemistry represents a pivotal shift in the chemical sciences, transitioning from a concept focused on pollution cleanup to a proactive framework for designing safer, more efficient chemical processes and products. This transformation was formally realized through the establishment of two key institutions: the Green Chemistry Institute (GCI) and the Presidential Green Chemistry Challenge Awards (GCCA). These institutions emerged in the 1990s as tangible manifestations of a growing consensus among chemists that environmental protection could be achieved most effectively through fundamental design rather than end-of-pipe remediation [29]. This institutional framework provided the infrastructure necessary to advance green chemistry from theoretical principles to practical applications across academic, industrial, and governmental sectors, creating a foundation for the ongoing evolution of sustainable chemistry practices worldwide [10].
Historical Context and Driving Forces
The development of green chemistry as a formal discipline occurred within a specific historical context marked by growing environmental awareness and regulatory evolution.
The Regulatory and Environmental Landscape
The 1960s through the 1980s witnessed a series of environmental milestones that set the stage for green chemistry's emergence:
- 1962: Rachel Carson's Silent Spring documented the detrimental effects of chemical pesticides, catalyzing public environmental consciousness [9] [29].
- 1970: The U.S. Environmental Protection Agency (EPA) was established, with its first major action being the ban of DDT and other chemical pesticides [29].
- 1980s: A paradigm shift occurred from pollution control to pollution prevention, with international bodies like the Organization for Economic Co-operation and Development (OECD) recommending cooperative changes to chemical processes [9] [29].
- 1988: The Office of Pollution Prevention and Toxics was established within the EPA, signaling an institutional commitment to preventative approaches [29].
This regulatory evolution created both the imperative and the infrastructure necessary for green chemistry's formalization.
Foundational Intellectual Framework
The intellectual foundation of green chemistry was codified in the 1990s through several key developments:
- 1991: The phrase "Green Chemistry" was officially coined by staff at the EPA Office of Pollution Prevention and Toxics [29].
- 1994: The first symposium, "Benign by Design: Alternative Synthetic Design for Pollution Prevention," was held in Chicago, sponsored by the ACS Division of Environmental Chemistry [29].
- 1998: Paul Anastas and John C. Warner co-authored Green Chemistry: Theory and Practice, outlining the 12 Principles of Green Chemistry that would become the field's philosophical guide [29].
These developments established the conceptual framework that would guide both the GCI and GCCA in their missions to advance sustainable chemistry.
Founding and Evolution of the Green Chemistry Institute (GCI)
Establishment as an Independent Nonprofit (1997)
The Green Chemistry Institute was founded in 1997 as an independent not-for-profit organization dedicated to promoting and advancing green chemistry [29]. The founding directors were:
- Dr. Joe Breen: A retired 20-year staff member of the EPA who became the Institute's first director [29].
- Dr. Dennis Hjeresen: A researcher at the Los Alamos National Laboratory who co-founded the institute [29].
The founding committee was chaired by Paul Anastas and included Joe Desimone (University of North Carolina), Bill Tumas (DuPont), and Sid Chao (Hughes Environmental) [29]. This diverse composition—spanning government, academia, and industry—reflected the institute's commitment to cross-sector collaboration from its inception.
Key Early Initiatives
In its initial years, the GCI launched several foundational programs:
- Green Chemistry & Engineering Conference (1997): Established to convene the growing green chemistry community and highlight GCCA winners [29]. The first conference attracted approximately 150 participants and was held at the National Academies headquarters in Washington, DC [29].
- First Education Summit (1999): Organized in collaboration with the University of Massachusetts, Boston, leading to a compendium of laboratory experiences illustrating green chemistry principles in undergraduate labs [29].
Integration into the American Chemical Society (2001)
Following Joseph Breen's passing in 2000, the EPA and ACS agreed to merge the GCI under the ACS umbrella [29]. In 2001, the GCI officially became part of the American Chemical Society, the world's largest professional scientific society [29]. This institutionalization within ACS signaled that green chemistry was gaining prominence as an essential part of chemistry's toolkit [29]. Nina McClelland, ACS Board Chair at the time, was instrumental in this arrangement, and Dennis Hjeresen was appointed Director of the ACS GCI [29].
Expansion and Sector-Specific Initiatives
Under ACS stewardship, the GCI expanded its influence through specialized industrial partnerships:
- 2005: The ACS GCI established its first Industrial Roundtable for the pharmaceutical industry to catalyze and enable green chemistry in chemical businesses [29].
- Subsequent roundtables were established for various sectors, including the Oilfield Chemistry Roundtable and Natural Polymers Consortium [29].
- These roundtables have awarded hundreds of thousands of dollars in green chemistry research grants and developed practical tools like the Reagent Guide to inform users about greener reagents for chemical transformations [30].
Table: Evolution of the Green Chemistry Institute
| Year | Milestone | Key Figures | Significance |
|---|---|---|---|
| 1997 | Founded as independent nonprofit | Joe Breen, Dennis Hjeresen, Paul Anastas | Established dedicated organization for green chemistry advancement |
| 1997 | Launched GC&E Conference | Paul Anastas, Joseph Breen | Created central gathering place for community knowledge-sharing |
| 1999 | First Education Summit | GCI & UMass Boston | Integrated green chemistry into academic curricula |
| 2001 | Merged with ACS | Nina McClelland, Dennis Hjeresen | Institutionalized within world's largest chemical society |
| 2005 | First Industrial Roundtable | ACS GCI | Established industry-academia collaboration model |
Creation and Impact of the Presidential Green Chemistry Challenge Awards
Establishment and Governance
The Presidential Green Chemistry Challenge Awards were established in 1995 when the EPA received support from President Bill Clinton to create an annual awards program highlighting scientific innovations in academia and industry that advanced Green Chemistry [29]. The program was designed to "recognize and promote innovative chemical technologies that prevent pollution and have broad applicability in the industry" [10].
Award Categories and Recognition Criteria
The GCCA recognizes innovations across multiple categories that demonstrate the application of green chemistry principles:
- Greener Synthetic Pathways
- Greener Reaction Conditions
- Design of Greener Chemicals
- Small Business
- Academic
- Specific Environmental Benefit: Climate Change (added in more recent years) [31]
Winning technologies must reduce or eliminate the use or generation of hazardous substances, demonstrate innovation, offer broad applicability, and provide economic benefits [32].
Evolution of Award-Winning Technologies
The GCCA has tracked the evolving focus of green chemistry applications over its history. Recent winners illustrate the field's expanding scope and sophistication:
Table: Representative Green Chemistry Challenge Award Winners (2020-2025)
| Year | Winner | Category | Innovation | Impact |
|---|---|---|---|---|
| 2025 | Keary M. Engle, Scripps Research | Academic | Air-stable nickel(0) catalysts | Replaces precious metals, eliminates need for energy-intensive inert-atmosphere storage [33] |
| 2025 | Merck & Co., Inc. | Greener Synthetic Pathways | Nine-enzyme biocatalytic cascade for islatravir | Replaced 16-step synthesis with single aqueous process [33] |
| 2025 | Future Origins | Specific Environmental Benefit: Climate Change | Non-palm C12/C14 fatty alcohols via fermentation | 68% lower global warming potential vs. palm kernel oil-derived equivalents [33] [32] |
| 2024 | Merck & Co., Inc. | Greener Synthetic Pathways | Continuous manufacturing process for KEYTRUDA | Improved efficiency in biologics manufacturing [31] |
| 2023 | Solugen | Greener Synthetic Pathways | Enzyme-based chemical production from renewable resources | Decarbonization of commodity chemicals [31] |
| 2022 | Cornell University (Song Lin) | Academic | Electrochemical synthesis of complex molecules | More efficient pharmaceutical intermediate production [31] |
| 2021 | Clemson University (Srikanth Pilla) | Academic | Nonisocyanate polyurethane (NIPU) foam | Eliminates hazardous isocyanates [31] |
| 2020 | Genomatica | Greener Synthetic Pathways | Biobased butylene glycol | Renewable replacement for petroleum-derived chemical [31] |
Methodologies and Experimental Protocols in Green Chemistry
Green chemistry methodologies have evolved significantly, with several approaches becoming particularly impactful. The following experimental protocols represent key methodologies that have received recognition through the GCCA program.
Enzyme Cascade Engineering for Pharmaceutical Synthesis
Representative Example: Merck's Nine-Enzyme Biocatalytic Cascade for Islatravir [33]
- Objective: Develop a streamlined, sustainable synthesis for the investigational antiviral islatravir
- Original Process: 16-step chemical synthesis requiring multiple isolations and organic solvents
- Green Chemistry Solution: Single biocatalytic cascade involving nine engineered enzymes
- Experimental Protocol:
- Enzyme Selection and Engineering: Identified and optimized enzymes through collaboration with Codexis using protein engineering techniques
- Reaction Optimization: Established optimal conditions for all nine enzymes to function sequentially in a single vessel
- Process Integration: Developed continuous processing without intermediate workups, isolations, or organic solvents
- Scale-up: Demonstrated process on 100 kg scale for commercial production
- Key Green Chemistry Principles: Waste prevention, safer solvents, design for energy efficiency, use of renewable feedstocks
Alternative Catalyst Development for Synthetic Chemistry
Representative Example: Air-Stable Nickel(0) Catalysts for Coupling Reactions [33]
- Objective: Develop practical, scalable nickel catalysts to replace precious metals
- Technical Challenge: Traditional nickel catalysts require energy-intensive inert-atmosphere handling
- Green Chemistry Solution: Novel nickel complexes combining high reactivity with air stability
- Experimental Protocol:
- Ligand Design: Synthesized specialized ligands that stabilize Ni(0) against oxidation while maintaining reactivity
- Electrochemical Synthesis: Developed alternative preparation method avoiding excess flammable reagents
- Activation Studies: Established conditions for generating catalytically active species under standard conditions
- Substrate Scope Evaluation: Tested performance across diverse carbon-carbon and carbon-heteroatom bond formations
- Key Green Chemistry Principles: Inherently safer chemistry, accident prevention, catalysis
The Scientist's Toolkit: Essential Research Reagent Solutions
Table: Key Reagent Solutions in Modern Green Chemistry
| Reagent/Technology | Function | Green Chemistry Advantage |
|---|---|---|
| Air-Stable Nickel Complexes [33] | Catalyze cross-coupling reactions | Replace precious metals (palladium); eliminate need for energy-intensive inert-atmosphere handling |
| Engineered Enzyme Systems [33] | Biocatalytic synthesis | Enable multistep transformations in single pot with high specificity; reduce solvent waste |
| Electrochemical Synthesis [33] | Reagent-free oxidation/reduction | Avoid stoichiometric oxidants/reductants; enable safer reaction conditions |
| Supercritical Water [31] | Reaction medium for biomass processing | Replace organic solvents; utilize renewable feedstocks |
| Non-Isocyanate Polyurethane Chemistry [31] | Polymer production | Eliminate use of highly toxic isocyanate starting materials |
| Bio-Based Feedstocks [31] | Renewable carbon sources | Reduce dependence on petroleum; utilize sustainable resources |
Institutional Impact and Current Landscape
Global Propagation and Recognition
The institutional foundation provided by the GCI and GCCA has facilitated global adoption of green chemistry principles:
- International Organizations: Green chemistry groups, journals, and conferences have launched worldwide, including:
- Academic Integration: Educational and research curricula became available at all levels, with the first Ph.D. program in green chemistry established at the University of Massachusetts Boston in 1997 [29].
- Scientific Recognition: Nobel Prizes in Chemistry in 2001 (asymmetric catalysis) and 2005 (metathesis) highlighted research areas aligned with green chemistry principles, further validating the field [29].
Current Challenges and Future Directions
Despite significant progress, challenges remain in the full adoption of green chemistry:
- Current Limitations: Nearly 90% of feedstocks used to make chemicals are still derived from fossil sources [29].
- Research Frontiers: Green chemists and engineers are increasingly focusing on:
- Circular Economy Models: Technologies like Pure Lithium Corporation's Brine to Battery method for closed-loop lithium-metal battery production [33]
- Carbon Utilization: Processes like Air Company's AIRMADE technology that converts CO₂ to sustainable aviation fuels [31]
- Toxics Reduction: Innovations like Cross Plains Solutions' SoyFoam that eliminates PFAS in firefighting foams [33]
- Measurement and Metrics: Continued development of standardized metrics to quantify the environmental and economic benefits of green chemistry innovations.
The GCI and GCCA continue to evolve to address these challenges, maintaining their role as central coordinating institutions for the global green chemistry community.
Institutionalization Timeline of Green Chemistry
Principles in Practice: Methodologies for Integrating Sustainable Chemistry into Pharmaceutical R&D
The evolution of the sustainable chemistry movement has fundamentally reshaped how chemists approach molecular synthesis. From the seminal publication of Rachel Carson's Silent Spring in 1962, which ignited public and scientific awareness of chemical pollution, to the U.S. Pollution Prevention Act of 1990, which established a national policy favoring pollution prevention over end-of-pipe treatment, the regulatory and philosophical landscape has progressively emphasized inherent hazard reduction [9] [18]. This trajectory culminated in the 1990s with the formalization of Green Chemistry as a distinct field. The U.S. Environmental Protection Agency's (EPA) staff coined the term "Green Chemistry," and the field was codified with the 1998 publication of Green Chemistry: Theory and Practice by Paul Anastas and John C. Warner, which introduced the Twelve Principles of Green Chemistry [9] [16] [18]. These principles provide a framework for designing chemical products and processes that reduce or eliminate the use and generation of hazardous substances.
A cornerstone of this philosophy is the redesign of synthetic methodologies to avoid traditional environmental and health burdens, with solvent reduction being a critical target. Conventional solution-phase synthesis relies heavily on volatile organic solvents, which account for a significant portion of the waste and energy footprint in sectors such as pharmaceuticals [34]. In this context, mechanochemistry—which uses mechanical force to drive reactions in the solid state or with minimal liquid—has emerged as a powerful solvent-free alternative. It aligns directly with multiple Green Chemistry principles, including waste prevention, safer solvents, and energy efficiency [35] [18]. This whitepaper provides a technical guide for researchers and drug development professionals seeking to integrate mechanochemistry into core synthetic strategies, framed within the historical context of the sustainable chemistry movement.
Mechanochemistry: Core Principles and Green Chemistry Alignment
Mechanochemistry involves the use of mechanical energy to induce chemical transformations, bypassing the need for molecular solvents to dissolve reactants. This energy is typically delivered through grinding, milling, or extrusion, leading to intimate mixing and increased reactivity between solid reagents [35]. The primary equipment includes:
- Mixer Mills: Jars containing ball bearings are rapidly shaken back and forth, generating impact and frictional forces.
- Planetary Ball Mills: Jars are spun to generate centrifugal force, pressing balls against reactants for larger gram-scale reactions.
- Simpler Tools: For some applications, a mortar and pestle or twin-screw extruders can be effective [35].
This approach offers a paradigm shift from traditional solvothermal methods, often resulting in shorter reaction times, room-temperature operation, and unique product selectivity [35]. The following diagram illustrates the core conceptual workflow of a mechanochemical synthesis.
The green chemistry advantages of this solvent-free approach are quantitative and significant, as shown in the following comparison of key environmental metrics.
Table 1: Quantitative Green Chemistry Advantages of a Model Mechanochemical Synthesis [36]
| Green Chemistry Metric | Traditional Solution-Based Method | Mechanochemical Method | Improvement Factor |
|---|---|---|---|
| Reaction Time | 0.5 - 4 hours | 10 minutes | ~3-24x faster |
| Temperature | Often requires heating | Room temperature | Energy saving |
| Solvent Volume | 1.5 mL per 0.5 mmol reactant | 0 mL (solvent-free) | 100% reduction |
| Catalyst/Additive | Often required (e.g., I₂, Cu, BiCl₃) | None (neat grinding) | 100% reduction |
| Isolated Yield | Up to 26% (in MeOH, no additive) | 92% | ~3.5x higher yield |
Detailed Experimental Protocols: A Case Study in Amination
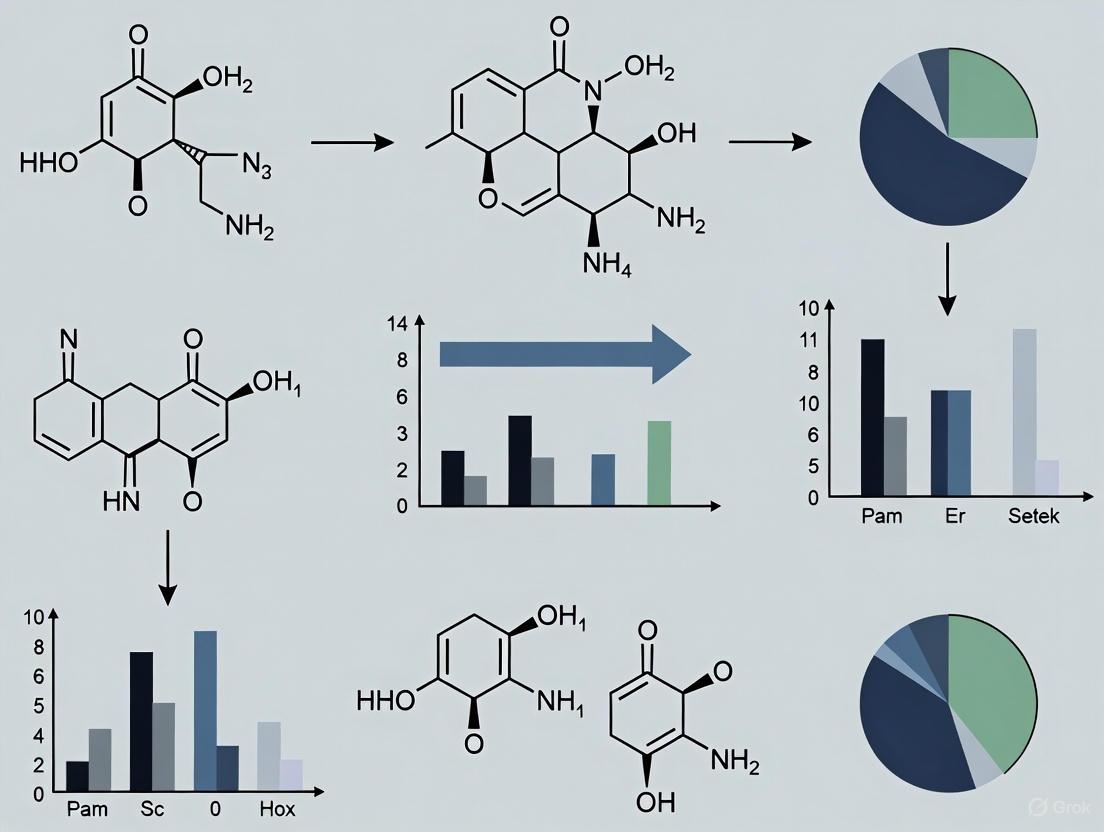
Recent literature provides robust, optimized protocols for implementing mechanochemistry. The following section details a specific case study: the solvent-free, regioselective amination of 1,4-naphthoquinones to synthesize biologically relevant 2-amino-1,4-naphthoquinones [36]. This reaction showcases the efficiency and practicality of the method.
Reaction Scheme and Optimization
The general reaction involves the coupling of a 1,4-naphthoquinone with an amine to form a 2-amino-1,4-naphthoquinone derivative.
Synthetic Scheme: 1,4-Naphthoquinone (1) + Amine (2) → 2-Amino-1,4-naphthoquinone (3)
The optimization process for this mechanochemical reaction is summarized in the table below, which systematically evaluates different parameters to achieve maximum yield.
Table 2: Optimization Table for the Model Mechanochemical Amination [36]
| Entry | Solvent | Solid Surface | Conditions | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| 1 | - | Neutral Alumina | Ball-milling (550 rpm) | 60 | - |
| 2 | - | Basic Alumina | Ball-milling (550 rpm) | 5 | 80 |
| 3 (Optimal) | - | Basic Alumina | Ball-milling (550 rpm) | 10 | 92 |
| 4 | - | Basic Alumina | Ball-milling (550 rpm) | 15 | 88 |
| 5 | - | Acidic Alumina | Ball-milling (550 rpm) | 10 | 28 |
| 6 | - | Silica / NaCl | Ball-milling (550 rpm) | 10 | Trace |
| 12-16 | Methanol / EtOH / etc. | - | Magnetic Stirring | 240 | 18-26 |
Step-by-Step Procedure
This protocol is adapted from the optimized conditions in [36].
- Loading Reactants: Place 1,4-naphthoquinone (1, 0.5 mmol) and the amine derivative (2, 0.5 mmol) into a 25 mL stainless-steel milling jar.
- Adding Grinding Media: Add basic alumina (1.5 g) as a solid surface to facilitate grinding and reaction. Introduce 7 stainless-steel balls (10 mm diameter) as the grinding media.
- Milling: Securely fasten the jar in a high-speed ball mill. Process the mixture at a frequency of 550 rpm for 10 minutes. The mill can be programmed to operate with a brief pause (e.g., 5 seconds) every 2.5 minutes to prevent overheating.
- Work-up & Isolation: After milling, open the jar. The product (3) is isolated from the basic alumina solid surface via column chromatography or trituration. A key advantage is the reusability of the basic alumina surface for subsequent reactions, enhancing the method's green credentials.
- Characterization: Characterize the final product using standard techniques, including ( ^1 \text{H} ) NMR, ( ^{13} \text{C} ) NMR, and HRMS, to confirm structure and purity [36].
The Scientist's Toolkit: Essential Research Reagent Solutions
Success in mechanochemical synthesis depends on the appropriate selection of equipment and materials. The following table details the key components of a mechanochemistry toolkit.
Table 3: Essential Materials and Equipment for Mechanochemical Research
| Item / Reagent | Function / Role in Synthesis | Technical Notes |
|---|---|---|
| Planetary Ball Mill | Applies mechanical energy via centrifugal force; ideal for gram-scale reactions and screening. | Allows control over rotational speed and time; jars available in various materials [35]. |
| Mixer Mill | Applies energy via high-frequency shaking; useful for smaller-scale, high-impact reactions. | Typically uses smaller jars and is efficient for rapid screening [35]. |
| Grinding Jars | Contain the reaction mixture. | Material choice (e.g., stainless steel, tungsten carbide, ceramic) depends on required chemical inertness and mechanical strength [36]. |
| Grinding Media (Balls) | Transmit mechanical energy to reactants through impact and friction. | Size, number, and material (e.g., steel, ceramic) are critical optimization parameters [36]. |
| Basic Alumina | Acts as a solid grinding auxiliary and heterogeneous base catalyst. | Promotes reactions in the absence of soluble catalysts; can be reused [36]. |
| Stainless-Sel Jars/Balls | Standard, robust equipment for most organic syntheses. | Provides high density for efficient energy transfer. pH of basic alumina suspension: ~8.01 [36]. |
The logical workflow for setting up and executing a mechanochemical experiment, from equipment selection to product isolation, is visualized below.
Quantitative Sustainability Assessment in Early-Stage Development
Integrating sustainability assessment during early-phase reaction design is crucial for guiding research toward genuinely greener processes [34]. Tools like DOZN 3.0, a quantitative green chemistry evaluator, enable researchers to measure their processes against the 12 Principles of Green Chemistry, providing a data-driven basis for claiming green credentials [37]. Furthermore, systematic reviews have identified over 50 methods suitable for early-phase sustainability assessment, emphasizing the importance of moving beyond single metrics to a multidimensional view that includes environmental, economic, and social impacts [34].
For the demonstrated mechanochemical amination, a preliminary assessment using common green chemistry metrics reveals a profoundly improved profile:
- E-factor (kg waste/kg product): Drastically reduced due to solvent elimination.
- Process Mass Intensity (PMI): Approaches the theoretical minimum, as only the mass of reactants and a reusable solid surface contribute.
- Energy Efficiency: Superior to heated solution-phase methods, as reactions proceed at room temperature in minutes [36] [34].
The adoption of solvent-free mechanochemistry represents a mature and practical response to the historical call for sustainable chemistry. As demonstrated, it offers a direct route to achieving the goals set forth by decades of environmental policy and green chemistry philosophy. The method provides tangible operational advantages—shorter reaction times, high yields, and simple work-ups—alongside compelling environmental benefits by design. For the pharmaceutical industry and other chemical sectors, integrating these techniques into core synthesis strategies is a critical step toward reducing the environmental footprint of research and production. The future of green chemistry lies in viewing its principles as a cohesive system, where improvements in one area, like solvent reduction, synergistically enhance others [16]. Mechanochemistry stands as a testament to this approach, enabling researchers to design syntheses that are not only efficient and elegant but also inherently sustainable.
Historical Context: The Rise of Green Chemistry
The paradigm shift towards using water as a benign solvent in organic chemistry is inextricably linked to the broader sustainable chemistry movement that gained significant traction in the late 20th century. For decades, industrial expansion occurred with minimal regard for environmental consequences, leading to increased pollution and resource depletion [18]. The 1962 publication of "Silent Spring" marked a pivotal moment, stimulating contemporary environmental awareness and prompting major governmental initiatives [18].
The formalization of green chemistry as a discipline emerged from regulatory frameworks, particularly the U.S. Pollution Prevention Act of 1990, which championed pollution elimination through improved design rather than end-of-pipe solutions [16]. By 1991, the EPA Office of Pollution Prevention and Toxics had launched a research grant program encouraging the redesign of chemical products and processes to reduce their environmental and health impacts [16]. This institutional support was crucial for fostering the fundamental research needed to challenge entrenched practices.
The field was codified with the establishment of the Twelve Principles of Green Chemistry in 1998, providing a clear set of design guidelines for developing benign chemical products and processes [18] [16]. A cornerstone of these principles is the emphasis on hazard prevention rather than management—addressing risk by minimizing intrinsic hazard rather than relying on exposure controls that can fail [16]. The introduction of the annual Presidential Green Chemistry Challenge Awards in 1996 further catalyzed the field by highlighting academic and industrial success stories [18] [16]. This historical trajectory, moving from reaction to prevention and from hazard management to intrinsic safety, created the necessary intellectual and regulatory environment for water to be re-evaluated as a viable reaction medium.
The Case for Water: From "Worst Enemy" to "Best Friend"
Historically, organic chemists regarded water as an enemy, adhering to the paradigm that "like dissolves like" and thus believing that hydrophobic organic compounds required hydrophobic organic solvents for effective reaction control [38] [39]. This notion was reinforced by the moisture sensitivity of many catalysts and reagents, making dry organic solvents the unquestioned norm [38]. However, the environmental and health drawbacks of traditional organic solvents became impossible to ignore. Many are associated with significant toxicity issues, including mutagenicity, teratogenicity, and carcinogenicity, while also posing risks of flammability and explosivity [38]. Their environmental impact, as volatile organic compounds (VOCs) contributing to air pollution and climate change, has led to increasingly stringent regulations like the Montreal Protocol and REACH [38].
In this context, water emerged as a safe, non-toxic, cheap, and environmentally benign alternative [38]. Seminal work by Breslow in 1980 demonstrated that water could not only facilitate organic transformations but, surprisingly, lead to remarkable rate enhancements and superior selectivities compared to organic solvents [38] [39]. This discovery challenged decades of established dogma and opened a new frontier for organic synthesis. The unique physical and chemical properties of water—the medium chosen by Nature for all of life's processes—began to be viewed not as a limitation, but as an opportunity for discovering new and unexpected chemical reactivity [38].
Table 1: Comparison of Water and Traditional Organic Solvents
| Property | Water | Traditional Organic Solvents |
|---|---|---|
| Environmental Impact | Benign, sustainable | Often toxic, persistent pollutants (VOCs) |
| Health & Safety | Non-toxic, non-flammable | Often toxic, flammable, explosive |
| Cost & Availability | Cheap, readily available | Often expensive, petroleum-based |
| Waste Generation | Minimal hazardous waste | Major source of hazardous chemical waste |
| Unique Properties | Hydrogen bonding, hydrophobic effect, high surface tension | Variable polarity, primarily solvation-based effects |
Fundamental Concepts: "On Water" vs. "In Water" Reactions
A critical understanding in this field is the distinction between "on water" and "in water" reactions, terms that describe different mechanistic phenomena and physical regimes.
"On Water" Reactions
The term "on water" was introduced by Sharpless in 2005 to describe reactions that experience "substantial rate acceleration when insoluble reactants are stirred in aqueous suspension" [38]. These are heterogeneous systems where the organic reactants are not dissolved but remain in a separate phase, with the reaction occurring at the oil-water interface [39]. A classic example is a [2σ + 2σ + 2π] cycloaddition reported by Sharpless, which reached completion in just 10 minutes "on water," while requiring 48 hours under neat conditions and over 18 hours in various organic solvents [38]. The reaction rate was conserved as long as heterogeneity was maintained, but slowed dramatically once a homogeneous mixture was achieved by adding methanol [38].
"In Water" Reactions
"In water" reactions refer to processes occurring in a homogeneous aqueous medium [39]. The seminal work of Rideout and Breslow in 1980 demonstrated a Diels-Alder reaction in water that proceeded 58-fold faster than in methanol and over 700-fold faster than in hydrocarbon solvents [38]. This acceleration was attributed to the hydrophobic effect, whereby non-polar reactants are pushed together as the water network seeks to minimize disruptive interactions with hydrophobic surfaces [38]. This was supported by the observation that adding salts (LiCl) to further decrease organic solubility (salting-out) increased the reaction rate, while guanidinium chloride, which reduces hydrophobic interactions, slowed the reaction [38].
Table 2: Key Characteristics of "On Water" and "In Water" Reaction Systems
| Characteristic | "On Water" Reactions | "In Water" Reactions |
|---|---|---|
| System Type | Heterogeneous | Homogeneous |
| Solubility of Reactants | Insoluble, separate phase | Soluble or dispersed at molecular level |
| Primary Mechanism | Interface effects, hydrogen bonding | Hydrophobic effect, solvation |
| Physical State | Suspension or emulsion | Solution |
| Key Demonstrations | Sharpless cycloaddition, Claisen rearrangement | Breslow's Diels-Alder, Suzuki coupling |
Quantitative Data and Experimental Evidence
The efficacy of aqueous reaction media is supported by substantial quantitative data demonstrating enhanced kinetics and selectivity across a range of important transformations.
Reaction Rate Enhancements
The dramatic acceleration of reactions in aqueous media is a consistent finding. As noted, the Diels-Alder reaction between cyclopentadiene and butenone showed a rate acceleration of more than 700-fold in water compared to hydrophobic solvents [38]. Analysis of the CAS Content Collection, the largest human-curated repository of scientific information, reveals a significant spike in journal and patent publications related to water-mediated organic reactions after 2010, indicating growing research and commercial interest [39]. While patent activity showed some volatility, there has been a strong resurgence since 2018, signaling renewed industrial confidence and technological advancement [39].
Prevalence of Reaction Types
Analysis of the scientific literature reveals which organic transformations have been most successfully adapted to aqueous media. Suzuki Coupling and Sonogashira Coupling are the most prevalent reactions found in the current literature [39]. The Diels-Alder reaction, a workhorse of organic synthesis, is also a leader, with its ubiquity meaning that numerous synthesis pathways can be made safer and more efficient [39]. The predominance of these C-C bond-forming reactions underscores a major research priority: developing fundamental reaction methodologies that operate efficiently in water [39].
Table 3: Key Organic Reactions with Demonstrated Success in Aqueous Media
| Reaction Name | Reaction Type | Key Applications | Performance in Water |
|---|---|---|---|
| Diels-Alder Cycloaddition | Pericyclic | Polymer synthesis, drug development | 10 min "on water" vs. 18+ h in organic solvents [38] |
| Suzuki Coupling | Cross-Coupling | Pharmaceuticals, fine chemicals | High prevalence in literature; efficient in aqueous media [39] |
| Sonogashira Coupling | Cross-Coupling | Drug development, molecular electronics | Dominant reaction in current research [39] |
| Claisen Rearrangement | Sigmatropic | Natural product synthesis | Accelerated under "on water" conditions [39] |
Experimental Protocols and Methodologies
General Protocol for an "On Water" Reaction
This protocol is adapted from the seminal Sharpless cycloaddition and similar heterogeneous reactions [38].
- Reaction Setup: In a round-bottom flask equipped with a magnetic stir bar, combine the organic reactants. The typical scale ranges from 0.1 to 2.0 mmol.
- Addition of Water: Add deionized water (typically 1.0–10.0 mL per mmol of limiting reactant) to the flask. The mixture will appear heterogeneous.
- Stirring: Stir the biphasic mixture vigorously (≥ 800 rpm) to create a fine suspension or emulsion and maximize the interfacial surface area.
- Reaction Monitoring: Monitor the reaction by TLC or GC-MS. The reaction temperature can be maintained at room temperature or heated, depending on the requirements.
- Work-up: Upon completion, extract the reaction mixture with a water-immiscible organic solvent (e.g., ethyl acetate or diethyl ether). Separate the organic layer.
- Purification: Dry the combined organic extracts over an anhydrous drying agent (e.g., MgSO₄), filter, and concentrate under reduced pressure. Purify the crude product using standard techniques like flash chromatography or recrystallization.
General Protocol for an "In Water" Reaction (Micellar Catalysis)
This protocol is adapted from methods using surfactants to solubilize organic compounds in water [38].
- Surfactant Solution Preparation: Prepare an aqueous solution of a designer surfactant (e.g., TPGS-750-M) at a concentration of 2–5% w/w in deionized water. Stir until the surfactant is fully dissolved.
- Reaction Setup: In a vial, sequentially add the surfactant solution, the organic substrates, and the catalyst. The reactants are solubilized within the hydrophobic cores of the micelles.
- Stirring: Stir the reaction mixture at moderate speed (300–500 rpm) to maintain homogeneity. The mixture should appear as a slightly opaque solution.
- Reaction Monitoring: Monitor the reaction by TLC or GC-MS. Many reactions proceed efficiently at or near room temperature.
- Work-up: Upon completion, cool the reaction mixture if necessary. Extract the product directly from the aqueous micellar solution with an organic solvent (e.g., heptane or ethyl acetate). Alternatively, the product may precipitate and be collected by filtration.
- Recycling: The aqueous surfactant solution can often be recycled for subsequent runs by simply adding new portions of substrates and catalyst.
Visualization of Concepts and Workflows
Diagram 1: The Water Paradigm Shift Logic
Diagram 2: Experimental Selection Workflow
The Scientist's Toolkit: Essential Reagents and Materials
Table 4: Key Research Reagent Solutions for Aqueous Chemistry
| Reagent/Material | Function/Description | Application Examples |
|---|---|---|
| Designer Surfactants (e.g., TPGS-750-M) | Forms nanomicelles in water that solubilize organic reactants and catalysts, enabling "in water" reactions. | Suzuki couplings, amide couplings, C-H functionalizations [38]. |
| Water-Compatible Ligands (e.g., sulfonated phosphines) | Modifies metal catalysts to be stable and active in aqueous environments, preventing decomposition. | Aqueous-phase hydrogenations, cross-coupling reactions [39]. |
| Water-Stable Lewis Acids | Acts as a catalyst in water, leveraging the unique ability of water to activate substrates via coordination. | Asymmetric aldol reactions, Diels-Alder reactions [38]. |
| Deionized/Degassed Water | The reaction medium itself; removing ions and oxygen prevents side reactions and catalyst deactivation. | Standard practice for all "in water" and "on water" reactions. |
The shift to utilizing water as a solvent represents a fundamental and necessary evolution in organic chemistry, firmly rooted in the principles of green chemistry. This paradigm is propelled by the compelling environmental and economic advantages of replacing toxic, petroleum-derived solvents with a safe, abundant, and benign alternative. The historical context of environmental regulation and the formalization of green chemistry principles provided the foundation for this shift, while striking experimental demonstrations of enhanced reaction rates and selectivities provided the proof of concept.
The future of this field is exceptionally promising. The standardization of sustainability metrics across research and industrial sectors is further elevating the value of aqueous synthetic approaches [39]. These methodologies are poised to revolutionize the development of pharmaceutical ingredients, fine chemicals, peptides, and complex heterocyclic compounds [39]. The benefits will be multifaceted, leading to the accelerated development of life-saving medications, improved synthetic efficiency, and a significant reduction in the environmental footprint of the chemical industry. By learning from and leveraging Nature's solvent, organic chemistry is finally aligning itself with the sustainable principles required for a healthier planet.
The emergence of green chemistry in the 1990s marked a paradigm shift in chemical research and pharmaceutical development, directly addressing the environmental consequences of chemical processes [40]. This movement, grounded in the 12 principles of green chemistry, compelled researchers to re-evaluate traditional solvents—a major source of pollution in the pharmaceutical and chemical industries [40]. Conventional extraction solvents like n-hexane, chloroform, and methanol, while effective, pose significant environmental and health hazards [40]. The ideal sustainable solvent would be nontoxic, biodegradable, derived from renewable feedstocks, and minimize waste generation [40].
Deep Eutectic Solvents (DES) represent a cornerstone innovation in this sustainable solvent revolution. First reported by Abbott et al. as a mixture of choline chloride and urea, DES are defined as mixtures of a Hydrogen Bond Acceptor (HBA) and a Hydrogen Bond Donor (HBD) that experience a significant melting point depression, resulting in a liquid eutectic mixture at room temperature [40] [41]. Their versatility, potentially biogenic origin, and tunable physicochemical properties have positioned DES as multi-task agents for a plethora of applications, from biomass valorization to the extraction of bioactive compounds, offering a promising alternative to conventional organic solvents [41] [42].
Fundamentals and Properties of Deep Eutectic Solvents
A DES is fundamentally a non-ideal eutectic mixture where the interaction between a HBA and a HBD leads to a profound depression of the freezing point, forming a dense network of hydrogen bonds that remains liquid at relatively low temperatures [40]. The term "deep" signifies this substantial deviation from ideal thermodynamic behavior [40].
Key Advantages and Disadvantages
DES offer a compelling profile for green extraction applications, though they are not without limitations.
Table 1: Advantages and Disadvantages of DES in Extraction Applications
| Advantages | Disadvantages |
|---|---|
| Low volatility and high thermal stability [40] | High viscosity, which can reduce mass transfer and fluidity [40] |
| Low toxicity and high biodegradability compared to conventional solvents [40] | High density, complicating separation in some processes [40] |
| Tunable physicochemical properties by selecting different HBA/HBD components [40] | Potential environmental impacts that are not yet fully understood [41] |
| Simple preparation with 100% atom economy and no required purification [40] | Some components, like choline chloride, are often derived from petrochemical feedstocks [41] |
| Excellent solvation power for a wide range of compounds [40] [43] | Limited data on recyclability and comprehensive life cycle assessment (LCA) [41] |
The Scientist's Toolkit: Common DES Components
The customizability of DES is one of their most powerful features. The table below catalogues key reagents used in the formulation of DES for extraction purposes.
Table 2: Key Research Reagent Solutions for DES Formulation
| Reagent | Type | Common Role in DES | Function & Notes |
|---|---|---|---|
| Choline Chloride (ChCl) | Quaternary ammonium salt | Hydrogen Bond Acceptor (HBA) | The most widely used HBA due to low cost, low toxicity, and biodegradability [40]. |
| DL-Menthol | Monoterpene | HBA or HBD | Often used in hydrophobic DES; provides a low-toxicity, bio-based option [44]. |
| Urea | Amide | Hydrogen Bond Donor (HBD) | A common HBD that forms low-melting mixtures with ChCl [41]. |
| Glycerol | Polyol | Hydrogen Bond Donor (HBD) | A viscous, non-toxic HBD from renewable sources [40]. |
| Lactic Acid | Carboxylic acid | Hydrogen Bond Donor (HBD) | A renewable HBD that can form low-viscosity DES [44]. |
| Malic Acid | Carboxylic acid | Hydrogen Bond Donor (HBD) | A natural fruit-derived acid used as an HBD [40]. |
| Caprylic Acid | Fatty acid | Hydrogen Bond Donor (HBD) | Used in carboxylic acid-based DES for extracting non-polar compounds [44]. |
| Water | - | Diluent / Component | Reduces viscosity, modulates polarity, and can act as an HBA or HBD [40]. |
DES Preparation and Experimental Protocols
The preparation of DES is a straightforward process that does not require complex purification steps, contributing to its green credentials and ease of implementation in the laboratory.
Standardized Workflow for DES Synthesis
The following diagram illustrates the general experimental workflow for the synthesis and validation of a Deep Eutectic Solvent.
Detailed Synthesis Methodology
The typical procedure for synthesizing a DES, such as the classic Choline Chloride:Urea (1:2) mixture, involves the following steps [41] [40]:
- Weighing: Precisely weigh the Hydrogen Bond Acceptor (e.g., Choline Chloride) and Hydrogen Bond Donor (e.g., Urea) in the desired molar ratio. For a ChCl:Urea (1:2) DES, this would be 1 mole of ChCl to 2 moles of Urea.
- Mixing: Combine the solid components in a round-bottom flask or a sealed container to prevent water absorption.
- Heating and Stirring: Heat the mixture with continuous magnetic stirring at a temperature of approximately 70-80°C. The mixture is maintained at this temperature until a clear, homogeneous liquid is formed, which typically takes 30-90 minutes depending on the components.
- Cooling and Storage: The resulting transparent liquid is then cooled to room temperature. If necessary, the DES can be stored in a desiccator to minimize moisture uptake.
The preparation is characterized by 100% atom economy, as no by-products are formed and no waste is generated, fulfilling key principles of green chemistry [40].
DES in Action: Extraction of Bioactive Compounds
DES have shown remarkable efficiency in the extraction of bioactive compounds from natural sources, often outperforming traditional organic solvents.
Molecular Mechanism of Extraction
The extraction process is governed by specific molecular interactions between the DES and the target compound. The following diagram details the mechanism by which a DES extracts a target bioactive molecule from a plant matrix.
The primary mechanism for extracting polar bioactive compounds, such as flavonoids and phenolic acids, is through hydrogen bonding [45]. The complex network of HBA and HBD in the DES interacts with the functional groups (e.g., -OH, -COOH) of the target molecule, effectively solubilizing it. Molecular dynamics simulations, as used in saffron bioactive extraction studies, reveal that the transfer of molecules from the aqueous phase to the DES phase is driven by van der Waals and electrostatic interactions [44]. The efficiency of this process is highly dependent on the structural and surface characteristics of both the DES and the bioactive molecule [44].
Quantitative Performance of DES in Extraction
The effectiveness of DES is demonstrated by their performance in extracting various bioactive compounds, often yielding better results than traditional solvents.
Table 3: Extraction Performance of DES for Selected Bioactive Compounds
| Target Compound | Source | DES Formulation (HBA:HBD) | Key Finding / Advantage |
|---|---|---|---|
| Crocin | Saffron | Choline Chloride-based [44] | High extraction efficiency under laboratory conditions [44]. |
| Flavonoids | Herbal Medicines | Various DES | Provides a sustainable and effective alternative to traditional, harmful solvents [45]. |
| Anthocyanins | Catharanthus roseus | Natural DES (NADES) | High extractability and stability, replacing conventional organic solvents [44]. |
| Collagen | Blue Shark Skin | Citric acid:Xylitol:Water NADES | Significantly improved extraction yields compared to traditional procedures without pre-treatment [42]. |
| Phenolic Compounds | Olive Leaf | Choline Chloride derivative-based | Novel green alternative solvents for efficient extraction [45]. |
| α-Carotene, β-Carotene, Zeaxanthin | Saffron | Caprylic acid + DL-Menthol (1:2) | Highest interaction energies (-134 to -144 kJ/mol) in MD simulations, indicating superior performance [44]. |
A Critical Perspective on the Greenness of DES
While DES are widely touted as green solvents, a critical examination of their entire life cycle is necessary to validate this claim. A major caveat is that not all DES are inherently green or sustainably produced [41]. For instance, choline chloride, the most ubiquitous HBA, is predominantly synthesized from petrochemical feedstocks (trimethylamine and ethylene oxide) in a carbon-emitting process [41]. Similarly, urea production also relies on fossil sources [41].
This underscores the importance of Life Cycle Assessment (LCA), a robust and holistic methodology for evaluating the true environmental footprint of DES systems from production to disposal [41]. As of late 2025, LCA studies on DES applications remain sparse, representing only about 0.3% of total DES research, indicating a significant knowledge gap [41]. Furthermore, the environmental fate and biodegradability of many DES are still scarcely explored [41]. Therefore, the scientific community must avoid overhyping the greenness of DES and instead pursue systematic investigations that incorporate LCA, techno-economic analysis, and a critical view of feedstock sources to ensure sustainability claims are evidence-based [41].
Deep Eutectic Solvents have undeniably emerged as a powerful and versatile class of solvents that align with the principles of the sustainable chemistry movement. Their tunability, low volatility, and potential for high biodegradability make them superior alternatives to many conventional solvents for the extraction of bioactive compounds, as evidenced by their successful application in recovering flavonoids, carotenoids, and proteins from various natural sources [45] [42] [44].
Future research should focus on bridging existing gaps to fully realize the potential of DES. Key areas include:
- Scaling Up: Transitioning from laboratory-scale success to industrially viable processes [45].
- Comprehensive Sustainability Metrics: Conducting more Life Cycle Assessments to quantify the environmental impacts of DES systems comprehensively [41].
- Developing Sustainable Formulations: Designing DES from purely renewable and sustainably sourced feedstocks [45].
- Advanced Recycling: Innovating efficient recycling protocols, such as using responsive DES (RDES) that can be switched between states for easier product separation and solvent reuse [46].
By addressing these challenges, DES can solidify their role as a cornerstone of green chemistry, enabling safer and more sustainable extraction processes in pharmaceutical development and beyond.
The transition to renewable feedstocks in the pharmaceutical industry represents a pivotal shift from the traditional linear production model of "extract, manufacture, use, and dispose" toward a regenerative, circular framework [47]. This transformation is rooted in the sustainable chemistry movement that emerged in response to growing environmental awareness and regulatory pressures throughout the late 20th century [16] [9]. The field officially coalesced in 1998 with the publication of the Twelve Principles of Green Chemistry by Paul Anastas and John Warner, which provided a clear set of design guidelines for reducing or eliminating the use and generation of hazardous substances in chemical design, manufacture, and application [16] [9].
The pharmaceutical industry faces particular pressure to transform its feedstock sourcing due to its historical reliance on petroleum-based raw materials and the significant environmental footprint of its manufacturing processes [47] [48]. A typical mammalian-cell bioprocess can consume tens of thousands of liters of water per kilogram of product and generate several tons of plastic waste per manufacturing campaign [47]. This linear approach has become increasingly unsustainable, driving the sector toward bio-based alternatives that align with the principles of green chemistry and circular economy models [47] [49].
Historical Foundations of Sustainable Chemistry
The conceptual foundations for renewable feedstocks in chemistry extend back to the earliest days of organic synthesis, when all chemical production relied on biological sources [50]. Before the exploitation of petroleum and coal deposits in the late 19th century, chemists derived starting materials exclusively from microorganisms, plants, and animals to synthesize complex natural products [50]. Early landmark syntheses—including von Baeyer's indigo synthesis and Ladenburg's coniine synthesis—depended entirely on these bio-based resources [50].
The modern sustainable chemistry movement gained formal structure with the Pollution Prevention Act of 1990, which established a new U.S. national policy favoring pollution prevention through improved design rather than treatment and disposal [16] [9]. By 1991, the EPA Office of Pollution Prevention and Toxics had launched a research grant program encouraging the redesign of chemical products and processes to reduce impacts on human health and the environment [16]. The introduction of the annual Presidential Green Chemistry Challenge Awards in 1996 helped draw attention to academic and industrial success stories, while the Twelve Principles of Green Chemistry, published in 1998, provided the field with a clear set of design guidelines [16] [9].
This historical evolution reflects a circular pattern: chemistry began with renewable resources, shifted to petrochemicals during the industrial revolution, and is now returning to biological feedstocks with advanced technological capabilities [50]. The pharmaceutical industry now stands at an inflection point where sustainability must evolve from incremental efficiency improvements to systemic regeneration through renewable feedstocks [47].
Renewable feedstocks for pharmaceutical applications can be categorized into several distinct classes based on their origin and chemical composition. Unlike petrochemicals, which offer limited chemical diversity primarily comprising alkanes, alkenes, and arenes, bio-based feedstocks often contain inherent functionalization (heteroatoms, stereocenters) that makes them valuable as advanced building blocks for synthesis [50].
Biomass-derived Feedstocks
- Lignocellulosic Biomass: Comprising cellulose, hemicellulose, and lignin from non-food agricultural residues (e.g., straw, hulls), forestry waste, and dedicated energy crops [47] [49]. These materials can be broken down into fermentable sugars and phenolic compounds for conversion into platform chemicals.
- Marine Biomass: Alginate from brown algae and chitin from crustacean exoskeletons represent valuable carbohydrate feedstocks [50]. These marine-derived polysaccharides can be processed into pharmaceutical excipients and chiral building blocks.
- Agricultural By-products: Waste streams from food processing, including dairy effluent, spent grains from breweries, and fruit/vegetable processing residues, contain high concentrations of carbohydrates, lipids, and amino acids that can be valorized as nutrient sources in fermentation processes [47].
CO₂ as a Feedstock
Carbon dioxide is being reimagined as a valuable carbon source rather than merely a waste product [49]. Through carbon capture and utilization (CCU) technologies, CO₂ can be converted into industrially relevant compounds:
- Methanol synthesis from CO₂ and hydrogen under high-pressure catalytic conditions
- Polycarbonate production incorporating CO₂ into polymer chains
- Electrochemical conversion to formate and other C1 building blocks
Biobased Platform Chemicals
Specific molecules derived from renewable resources that serve as intermediates for pharmaceutical synthesis:
- Lactic acid from corn or bagasse fermentation, used to produce polylactic acid (PLA) resins for packaging [51]
- Isobutanol from sugar fermentation, replacing propylene-derived counterparts in coating resins and solvents [51]
- Paraxylene from wood chip feedstocks, enabling production of 100% biobased polyethylene terephthalate (Bio-PET) [51]
Table 1: Classification of Major Renewable Feedstocks for Pharmaceutical Applications
| Feedstock Category | Specific Examples | Primary Components | Pharmaceutical Applications |
|---|---|---|---|
| Lignocellulosic Biomass | Wood chips, agricultural residues, dedicated energy crops | Cellulose, hemicellulose, lignin | Platform chemicals, fermentation nutrients, excipients |
| Carbohydrate-Rich Biomass | Corn, sugarcane, sugar beet, cassava | Starch, sucrose, invert sugars | Fermentation feedstocks for APIs, solvents, biopolymers |
| Lipid-Rich Biomass | Plant oils, algal lipids, waste cooking oil | Triglycerides, fatty acids, phospholipids | Softgel capsules, drug delivery systems, formulation aids |
| Protein-Rich Biomass | Dairy waste, plant meals, microbial biomass | Amino acids, peptides, enzymes | Nutrient sources, chiral pool, catalytic applications |
| Marine Biomass | Algae, crustacean shells, fish processing waste | Alginate, chitin, chitosan, carrageenan | Drug delivery systems, wound healing materials, excipients |
| Carbon Dioxide | Industrial emissions, direct air capture | CO₂ | C1 building blocks, polymers, solvents |
Technological Pathways for Feedstock Conversion
The transformation of renewable feedstocks into pharmaceutical intermediates employs diverse technological approaches, ranging from traditional bioconversion to emerging catalytic processes.
Thermochemical Conversion
Hydrothermal liquefaction (HTL) employs elevated temperatures (200-350°C) and high pressures (10-25 MPa) in a water-rich environment to depolymerize complex biomass into bio-crude oil [49]. This method is particularly advantageous for processing wet feedstocks like algae without requiring energy-intensive drying steps. The resulting bio-crude can be refined into a range of chemicals compatible with existing pharmaceutical manufacturing infrastructure.
Biological Conversion
Fermentation and biocatalysis leverage microorganisms or isolated enzymes to convert biomass components into targeted molecules:
- Primary metabolites (ethanol, lactic acid, citric acid) produced through native microbial pathways
- Secondary metabolites (antibiotics, specialty chemicals) via engineered strains
- Asymmetric synthesis using engineered enzymes for chiral intermediate production
Catalytic Conversion
Advanced catalysis plays a crucial role in the efficient transformation of renewable feedstocks:
- Engineered enzymes (cellulases, hemicellulases) for selective depolymerization of biomass components [49]
- Metal-organic frameworks (MOFs) with tunable structures for precise control of reaction pathways in CO₂ utilization and biomass conversion [49]
- Bifunctional catalysts containing multiple active sites for simultaneous complementary reactions such as lignin depolymerization and bio-oil hydrogenation [49]
Diagram 1: Technological pathways for converting renewable feedstocks into pharmaceutical products
Experimental Protocol: Hydrothermal Liquefaction of Lignocellulosic Biomass
Objective: Convert lignocellulosic biomass into bio-crude oil for pharmaceutical intermediate production.
Materials and Equipment:
- Feedstock: Dried, milled agricultural residues (e.g., corn stover, wheat straw)
- High-pressure reactor system with temperature and pressure controls
- Water as reaction medium
- Nitrogen gas for inert atmosphere
- Filtration apparatus
- Solvents for product extraction (dichloromethane or acetone)
Procedure:
- Feedstock Preparation: Reduce biomass to particle size of 1-2 mm using a laboratory mill to enhance heat and mass transfer during processing.
- Reactor Loading: Charge the high-pressure reactor with biomass and water at a solid-to-liquid ratio of 1:10.
- Reaction Conditions: Pressurize system with nitrogen to 2 MPa, then heat to target temperature (250-300°C) with continuous stirring at 500 rpm. Maintain reaction for 30-60 minutes.
- Product Recovery: After reaction, cool reactor rapidly to room temperature. Collect aqueous and solid phases by filtration.
- Bio-crude Extraction: Separate bio-crude from aqueous phase using solvent extraction with dichloromethane (3 × 50 mL portions). Combine organic extracts and evaporate solvent under reduced pressure.
- Analysis: Characterize bio-crude yield, elemental composition, and chemical profile using GC-MS, FTIR, and elemental analysis.
Key Parameters for Optimization:
- Temperature: 200-350°C
- Pressure: 10-25 MPa
- Reaction time: 15-90 minutes
- Catalyst addition (optional): Heterogeneous acid catalysts (zeolites) to improve bio-crude quality
Implementation Framework for Pharmaceutical Manufacturing
The integration of renewable feedstocks into pharmaceutical manufacturing requires a systematic approach addressing technical, economic, and regulatory considerations.
Circular Biomanufacturing Framework
Circular biomanufacturing represents a paradigm shift from linear "take-make-waste" models to regenerative systems that continuously recycle and renew resources [47]. This framework rests on four interdependent pillars:
- Resource Efficiency: Focuses on reducing material, energy, and water intensity per unit of product through in-line recovery systems, high-solid fermentations, and continuous operations [47].
- Waste Valorization: Converts process by-products (cell debris, spent media, off-gases) into value-added materials such as fertilizers, biofuels, or secondary metabolites [47].
- Renewable Inputs: Favors renewable carbon sources including agricultural residues, waste biomass, or captured CO₂ over petrochemical precursors [47].
- Regenerative Process Design: Integrates equipment designed for disassembly and material recovery, couples manufacturing with renewable energy microgrids, and embeds real-time analytics for continuous optimization [47].
Metrics for Assessment
Quantitative metrics are essential for evaluating the sustainability and circularity of renewable feedstock implementation:
- E-factor: Mass of waste per mass of product, with lower values indicating higher process efficiency [47]
- Carbon Circularity Index: Fraction of carbon recycled within the process [47]
- Water Reuse Ratio: Volume of recycled water relative to total consumption [47]
- Atom Economy: Molecular mass of desired product relative to total molecular mass of all products [50]
Table 2: Comparative Analysis of Feedstock Options for Pharmaceutical Manufacturing
| Parameter | Petrochemical Feedstocks | First-Generation Bio-based | Second-Generation Bio-based | CO₂ Utilization |
|---|---|---|---|---|
| Carbon Source | Fossil reserves (finite) | Food crops (sugarcane, corn) | Non-food biomass (waste, residues) | Industrial emissions, air |
| Feedstock Cost | Subject to oil price volatility | Higher, commodity-dependent | Lower potential (waste valorization) | Low (with capture) |
| Environmental Impact | High carbon emissions, depletion | Land use, water consumption, food competition | Lower carbon footprint, waste reduction | Carbon negative potential |
| Technical Maturity | Established, optimized | Commercial scale | Pilot to demonstration scale | Early R&D to pilot |
| Chemical Diversity | Limited (hydrocarbons) | Moderate (carbohydrates) | High (varied functionalities) | Limited (C1 building blocks) |
| Integration Challenge | N/A (established) | Moderate (modification needed) | High (new infrastructure) | Very high (novel processes) |
| Regulatory Pathway | Established | Established, with sustainability criteria | Emerging, case-by-case | Nascent framework |
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Research Reagents for Renewable Feedstock Conversion
| Reagent/Material | Function | Application Example | Sustainability Consideration |
|---|---|---|---|
| Deep Eutectic Solvents (DES) | Customizable, biodegradable solvents for extraction | Metal recovery from e-waste, biomass processing | Low toxicity, renewable components (e.g., choline chloride) [52] |
| Metal-Organic Frameworks (MOFs) | Tunable catalysts with high surface area | CO₂ conversion, selective catalysis | Reusable, high selectivity reduces waste [49] |
| Engineered Enzymes (Cellulases, Hemicellulases) | Selective biomass depolymerization | Lignocellulose hydrolysis to fermentable sugars | Biodegradable, high specificity, mild conditions [49] |
| Silver Nanoparticles | Catalysis, antimicrobial applications | Nanoparticle synthesis in aqueous media [52] | Water-based synthesis reduces solvent use [52] |
| Choline Chloride-Urea Mixtures | Deep eutectic solvent formation | Biomass fractionation, lignin extraction | Renewable, biodegradable, low toxicity [52] |
| Bifunctional Catalysts | Multiple simultaneous reactions | Lignin depolymerization with simultaneous hydrogenation | Process intensification, reduced energy consumption [49] |
Current Applications and Case Studies
The pharmaceutical industry has begun implementing renewable feedstocks in both upstream synthesis and downstream processing, with several notable successes.
Feedstock Circularity in Pharmaceutical Production
Penn State Dairy Waste Conversion: Researchers developed an integrated biomanufacturing platform that converts dairy waste streams into usable carbon and nitrogen sources for microbial fermentation, drastically reducing the need for refined sugars or peptones [47]. This approach simultaneously addresses waste management and raw material sourcing challenges.
California Agricultural Waste Valorization: Regional initiatives are redirecting farm waste, including almond hulls, straw, and crop residues, into bio-based production pipelines supporting both energy and pharmaceutical applications [47]. These projects reduce landfill burden while creating value-added pathways for agricultural waste.
Process Circularity Implementation
Buffer Recovery Systems: Closed-loop ultrafiltration and diafiltration systems now enable reuse of process buffers while maintaining GMP-compliant purity [47]. Recent pilot-scale demonstrations show that buffer reuse can reduce total water consumption by 40-60% without compromising quality metrics.
Single-Use Technology Alternatives: While single-use bioreactors and filtration systems have enabled manufacturing flexibility and sterility, they have also entrenched a "take-make-waste" paradigm [47]. The industry is now developing biobased alternatives to single-use plastics and implementing recycling programs for these components.
Industrial Case Studies
Pfizer's Net-Zero Journey: Pfizer reduced CO₂ emissions by 60% at its Puurs, Belgium plant through digitization, solar power integration, and green manufacturing techniques [48].
Novartis's Renewable Drive: Novartis has converted its European operations to renewable power, investing in wind farms and on-site solar grids to decarbonize manufacturing [48].
Future Perspectives and Research Directions
The continued adoption of renewable feedstocks in pharma will be shaped by several emerging technological and methodological trends.
Emerging Technological Frontiers
- Mechanochemistry: Uses mechanical energy through grinding or ball milling to drive chemical reactions without solvents, reducing environmental impacts and enhancing safety [52].
- AI-Guided Reaction Optimization: Machine learning models trained to evaluate reactions based on sustainability metrics (atom economy, energy efficiency, toxicity) can suggest safer synthetic pathways and optimal reaction conditions [52] [53].
- In-Water and On-Water Reactions: Leverage water's unique properties (hydrogen bonding, polarity, surface tension) to facilitate chemical transformations, replacing toxic organic solvents [52].
- Waste Valorization Advanced Platforms: Integrated systems that combine multiple waste streams (agricultural, industrial, electronic) to create comprehensive circular economies [52].
Policy and Regulatory Landscape
The Sustainable Chemistry Research and Development Act of 2021 establishes a federal government-wide effort to enable U.S. leadership in innovation, commercialization, and adoption of safer, more sustainable chemicals and materials [54]. This legislation recognizes that despite more than 20 years of effort, green and sustainable chemistry remains niche in both academic chemistry and the chemical industry due to barriers including limited policy incentives, coordinated government leadership, and targeted research funding [54].
Future regulatory developments will likely increasingly tie research and industrial incentives to circularity metrics and carbon reduction targets [47]. Pharmaceutical companies may face escalating requirements to demonstrate sustainable sourcing and manufacturing practices as part of the drug approval process [48].
Implementation Roadmap
A phased approach to renewable feedstock integration suggests the following trajectory:
- Short-term (1-3 years): Focus on waste valorization, solvent substitution, and energy efficiency improvements
- Medium-term (3-7 years): Implementation of continuous manufacturing, advanced buffer recovery, and renewable energy integration
- Long-term (7-15 years): Development of fully circular biomanufacturing facilities with closed-loop material flows and industrial symbiosis networks
The transition from petrochemical to renewable feedstocks represents both a return to chemistry's biological origins and a forward-looking transformation enabled by advanced technologies [50]. This shift aligns with the historical development of green chemistry principles while addressing contemporary pressures including climate change, resource scarcity, and evolving regulatory requirements [16] [9] [54].
For pharmaceutical researchers and manufacturers, renewable feedstocks offer a pathway to reduce environmental impact, enhance supply chain resilience, and meet stakeholder expectations for sustainable practices [47] [48]. While technical and economic challenges remain, continued innovation in conversion technologies, coupled with supportive policy frameworks, is accelerating adoption across the sector.
The ultimate goal is a transition from linear efficiency—doing less harm—toward a regenerative logic that creates positive value across the pharmaceutical product life cycle [47]. By redesigning materials and processes according to biological principles, the industry can simultaneously advance human health and environmental sustainability, fulfilling the original promise of the green chemistry movement.
The sustainable chemistry movement, formally articulated by Paul Anastas and John Warner through the 12 Principles of Green Chemistry in 1998, established a foundational framework for designing chemical products and processes that reduce or eliminate hazardous substances [16] [18]. This philosophy emerged from earlier environmental protection efforts, including the U.S. Pollution Prevention Act of 1990, which marked a strategic shift from pollution control to pollution prevention through improved design [16]. For decades, the principles of atom economy, safer solvents, and waste prevention have provided a conceptual roadmap, yet their full implementation has often been hampered by the immense complexity of chemical and biological systems.
The contemporary convergence of artificial intelligence (AI) with these established sustainability goals represents a transformative evolution. AI provides the computational intelligence to navigate this complexity, making the principles of green chemistry more actionable and scalable than ever before. In predictive toxicology, AI models can now forecast potential health and environmental hazards of chemicals before they are synthesized, aligning with the preventive nature of green chemistry. Similarly, in reaction engineering, AI algorithms can design synthetic pathways that prioritize energy efficiency, renewable feedstocks, and reduced waste generation. This technical guide explores how AI is digitally transforming these fields, operationalizing decades of sustainable chemistry theory into practical, data-driven methodologies for researchers and drug development professionals.
AI in Predictive Toxicology
Market Landscape and Core Technologies
The integration of AI into predictive toxicology is experiencing rapid growth, driven by the need for faster, cost-effective, and more ethical drug development and chemical safety assessment. The global market for AI in predictive toxicology, valued at an estimated $635.8 million in 2025, is projected to reach $3,925.5 million by 2032, expanding at a strong compound annual growth rate (CAGR) of 29.7% [55] [56].
Table 1: Global AI in Predictive Toxicology Market Snapshot (2025-2032)
| Metric | 2025 Estimate | 2032 Projection | CAGR (2025-2032) |
|---|---|---|---|
| Market Size | USD 635.8 Million | USD 3,925.5 Million | 29.7% |
| Leading Technology Segment | Classical Machine Learning (56.1% share) | ||
| Dominant Region | North America (40.3% share) | ||
| Fastest-Growing Region | Asia Pacific (21.5% share) |
This growth is underpinned by several key technological segments:
- Classical Machine Learning: Dominating the market with a 56.1% share in 2025, this segment includes interpretable models like Support Vector Machines (SVMs), Random Forests, and decision trees [56]. Their success is attributed to their effectiveness with structured toxicology datasets, relative computational efficiency, and the transparency they offer—a valued feature for regulatory acceptance.
- Deep Learning: Utilizing complex neural networks, deep learning models excel at identifying intricate patterns from high-dimensional data, such as molecular graphs or high-throughput screening data [57] [58].
- Physics-based and Molecular Modelling: This segment integrates AI with computational chemistry and molecular simulations to predict toxicological outcomes based on fundamental physical principles and protein-ligand interactions [55].
Experimental Protocol: Developing a ToxCast-Based AI Prediction Model
The following protocol outlines a standard methodology for developing an AI-based toxicity prediction model, leveraging large-scale public data sources like the U.S. EPA's ToxCast program, one of the most widely used toxicological databases for this purpose [58].
Table 2: Essential Research Reagents and Computational Tools for AI Toxicology
| Reagent / Tool | Function in Experimental Workflow |
|---|---|
| ToxCast Database | Provides a large-scale, high-throughput screening data source for hundreds of assays and molecular endpoints, serving as the primary training data [58]. |
| Molecular Descriptor/Fingerprint Kits | (e.g., RDKit, Dragon). Standardizes molecular structures into numerical or binary vectors representing key physicochemical properties or structural features. |
| Machine Learning Libraries | (e.g., Scikit-learn, TensorFlow, PyTorch). Provides the algorithmic backbone for building, training, and validating classical ML and deep learning models. |
| Model Interpretation Tools | (e.g., SHAP, LIME). Provides post-hoc explanations for model predictions, crucial for building scientific and regulatory trust [56]. |
Step 1: Data Acquisition and Curation
- Download ToxCast assay data and chemical structures for the compounds of interest from the EPA's website. The data typically includes results from hundreds of in vitro assays targeting a wide range of biological endpoints (e.g., nuclear receptor signaling, stress response pathways) [58].
- Critical Step: Perform rigorous data cleaning and pre-processing. This involves handling missing values, correcting data types, and removing low-variance assays. The quality of the input data is the single greatest factor influencing model performance.
Step 2: Feature Engineering and Molecular Representation
- For each chemical structure, compute a set of numerical features (descriptors). Common choices include:
- Molecular Fingerprints: Hashed binary vectors representing the presence or absence of specific substructures.
- Physicochemical Descriptors: Quantitative values for properties like molecular weight, logP (hydrophobicity), polar surface area, etc.
- Graph Representations: For graph neural networks (GNNs), represent molecules as graphs where atoms are nodes and bonds are edges [58].
Step 3: Model Training and Validation
- Split the curated dataset into a training set (e.g., 80%) and a hold-out test set (e.g., 20%).
- Train a suite of machine learning models (e.g., Random Forest, Gradient Boosting, Neural Networks) on the training set to predict a specific ToxCast endpoint (e.g., estrogen receptor antagonism).
- Optimize model hyperparameters using cross-validation on the training set to prevent overfitting.
- Validation: Evaluate the final optimized model on the hold-out test set. Report standard performance metrics such as Accuracy, Sensitivity, Specificity, and Area Under the Receiver Operating Characteristic Curve (AUC-ROC).
Step 4: Model Interpretation and Application
- Use interpretation tools like SHAP (SHapley Additive exPlanations) to identify which molecular features or substructures the model deems most important for its prediction. This step is critical for transforming a "black box" prediction into an interpretable, actionable hypothesis for chemists and toxicologists [58].
- The validated model can then be deployed to screen virtual libraries of novel compounds for potential toxicity early in the design phase.
AI for Sustainable Reaction Pathway Design
Aligning Synthesis with Green Chemistry Principles
Sustainable reaction engineering focuses on designing chemical processes that minimize environmental impact, a goal deeply aligned with the 12 Principles of Green Chemistry [16]. AI acts as a powerful enforcer of these principles by allowing researchers to computationally evaluate and optimize reactions for factors like atom economy, energy efficiency, and the use of safer solvents and renewable feedstocks before any lab work begins. For instance, AI can help replace rare or toxic catalysts with earth-abundant and benign alternatives, such as the iron-based catalyst developed by Fañanás et al. for converting methane into chemical building blocks, a process that uses LED light and avoids precious metals [59].
Experimental Protocol: In Silico Design of a Sustainable Catalytic Reaction
This protocol describes a computational approach for designing a sustainable catalytic reaction pathway using AI and multi-objective optimization, reflecting the methodologies employed by leading research groups in the field [59] [60].
Table 3: Key Reagents and Computational Tools for Sustainable Pathway Design
| Reagent / Tool | Function in Experimental Workflow |
|---|---|
| Reaction Database | (e.g., USPTO, Reaxys). Provides a corpus of known chemical reactions for training generative AI and predicting reaction outcomes. |
| Quantum Chemistry Software | (e.g., Gaussian, ORCA). Calculates electronic structure properties to estimate reaction energies, barriers (kinetics), and spectroscopic data. |
| Machine Learning Force Fields | Accelerates molecular dynamics simulations, allowing for the rapid exploration of catalyst behavior and reaction mechanisms. |
| Process Modeling & LCA Software | (e.g., Aspen Plus, OpenLCA). Integrates with reaction data to estimate overall environmental impact (e.g., E-factor, Carbon Footprint). |
Step 1: Define Optimization Objectives and Constraints
- Formulate the reaction design as a multi-objective optimization problem. Objectives typically include:
- Maximize Yield/Selectivity: The primary goal for reaction efficiency.
- Minimize Energy Input: Favor reactions that proceed under mild temperature and pressure.
- Minimize Environmental Impact: Use calculated metrics like the E-factor (kg waste / kg product) or incorporate life cycle assessment (LCA) data early in the design [60].
- Use Benign Solvents/Catalysts: Constrain the search space to solvents from a "green" list (e.g., water, ethanol) and catalysts free of heavy metals.
Step 2: Reaction Space Exploration with Generative AI
- Use a generative AI model (e.g., a variational autoencoder or a generative adversarial network) to propose novel retrosynthetic pathways or catalyst structures.
- The model is trained on large datasets of chemical reactions (e.g., the USPTO patent database) and learns the complex rules of chemical reactivity.
- Application: For a target molecule, the AI proposes multiple synthetic routes. Each proposed reaction step is then scored against the objectives from Step 1.
Step 3: High-Throughput In Silico Screening
- For the most promising candidate reactions and catalysts identified in Step 2, perform rapid in silico screening.
- Use machine learning models, trained on quantum mechanics calculations, to predict key performance indicators like catalytic activity, selectivity, and stability.
- For catalyst design, graph neural networks can predict the binding affinities of potential catalyst molecules to substrates.
Step 4: Multi-objective Optimization and Pathway Selection
- A multi-objective optimization algorithm (e.g., NSGA-II) is used to navigate the trade-offs between the competing objectives (e.g., high yield vs. low energy cost).
- The output is a Pareto front—a set of candidate reaction pathways where no single objective can be improved without worsening another.
- The chemist can then select the most practically viable pathway from this front for experimental validation in the lab.
The digital transformation of chemistry through AI is fundamentally strengthening the application of green chemistry principles. By integrating predictive toxicology early in the molecular design process, AI helps realize the foundational tenet of pollution prevention [16]. Similarly, AI-driven reaction pathway design directly promotes atom economy, reduced energy intensity, and the use of safer materials [59] [60]. This synergy is moving the chemical industry from a paradigm of risk management to one of intrinsic hazard minimization.
Future progress hinges on overcoming key challenges, notably the limited availability of high-quality, standardized toxicological data and the need for clearer regulatory frameworks for accepting AI-based predictions [55] [56]. The next frontier involves developing more explainable AI (XAI) models that provide transparent rationales for their predictions, thereby building trust among scientists and regulators [58]. Furthermore, the full integration of AI-predicted chemical properties and reaction outcomes with quantitative lifecycle assessment (LCA) tools will enable a truly holistic evaluation of sustainability, from molecular design to end-of-life. As these technologies mature, the vision of a sustainable, efficient, and AI-driven chemical industry, long-held by the green chemistry movement, is poised to become a widespread reality.
The sustainable chemistry movement, emerging prominently in the 1990s, represents a paradigm shift from traditional "take-make-waste" industrial models toward a framework centered on pollution prevention and resource efficiency [61] [62]. This evolution was driven by the recognition that end-of-pipe pollution control is inherently less effective than designing chemical processes that minimize hazard and waste from the outset. Green chemistry, formally articulated through Twelve Principles by Anastas and Warner, established the foundational goal of reducing or eliminating the use and generation of hazardous substances [63]. A central tenet of this philosophy is the preference for catalytic processes over stoichiometric reactions, coupled with the strategic design of syntheses to maximize atom economy [62] [64].
The concept of atom economy, introduced by Barry Trost, quantitatively measures the efficiency of a chemical reaction by calculating the proportion of atoms from the starting materials that are incorporated into the final desired product [64]. A higher atom economy indicates less waste generation. This principle, along with the drive for energy efficiency, has moved from a peripheral concern to a central design criterion in modern chemical research, particularly in energy-intensive sectors like pharmaceuticals and petrochemicals [62] [65]. The field is now transitioning from a singular focus on green chemistry to a more comprehensive sustainable chemistry approach that incorporates systems and life cycle thinking, considering environmental, societal, and economic impacts across a molecule's entire lifespan [61] [62]. This review explores how catalytic strategies serve as the cornerstone for achieving energy efficiency and high atom economy, enabling a more sustainable chemical industry.
Foundational Principles: Atom Economy and Catalytic Efficiency
Quantifying Synthetic Efficiency: Atom Economy
Atom economy provides a simple yet powerful metric for evaluating the potential waste of a chemical reaction before it is even conducted. It is calculated as the molecular weight of the desired product divided by the sum of the molecular weights of all reactants, expressed as a percentage [64]. This concept is distinct from reaction yield, which measures the actual amount of product obtained; a reaction can have a high yield but a poor atom economy if significant waste by-products are generated.
Reactions can be broadly categorized by their inherent atom economy:
- High Atom Economy: Addition reactions and rearrangement reactions typically feature high atom economy, as they incorporate most or all reactant atoms into the final product [64]. The Diels-Alder cycloaddition, for instance, is a classic example of a 100% atom-economical reaction [64].
- Variable Atom Economy: Substitution and elimination reactions often generate stoichiometric amounts of by-products, resulting in lower atom economy [64].
The following table summarizes the atom economy of common organic reaction types:
Table 1: Atom Economy of Fundamental Organic Reactions
| Reaction Type | General Description | Typical Atom Economy | Example |
|---|---|---|---|
| Addition | Two molecules combine to form a single product. | High to Excellent | Diels-Alder, Catalytic Hydrogenation [64] |
| Rearrangement | Redistribution of atoms within a molecule to form an isomer. | Excellent (100%) | Claisen, Beckmann rearrangements [64] |
| Substitution | An atom or group is replaced by another. | Variable, often Moderate to Low | Nucleophilic substitution (e.g., SN1, SN2) |
| Elimination | A molecule loses atoms to form a multiple bond. | Poor to Moderate | Dehydrohalogenation (e.g., formation of an alkene) |
The Catalytic Imperative in Green Chemistry
The principle that "catalytic reagents are superior to stoichiometric reagents" is a pillar of green chemistry [62]. Catalysts enhance energy efficiency and atom economy by:
- Lowering Activation Energy: Catalysts provide an alternative reaction pathway with a lower energy barrier, enabling reactions to proceed under milder temperature and pressure conditions, which significantly reduces energy consumption [63].
- Enhancing Selectivity: By precisely controlling the reaction pathway, catalysts minimize side reactions and the formation of unwanted by-products, thereby improving atom economy and reducing purification burdens [66].
- Enabling New Pathways: Catalysis opens doors to novel, more direct synthetic routes that are not feasible with stoichiometric reagents, often leading to shorter syntheses with fewer steps and less waste [66].
Catalytic Strategies for Energy-Efficient Synthesis
Advanced Catalytic Materials
Innovations in catalyst design are crucial for improving the sustainability of chemical processes. Researchers are developing diverse catalytic materials to replace energy-intensive and waste-generating traditional methods.
Table 2: Advanced Catalytic Materials for Energy Efficiency
| Catalyst Type | Key Features | Applications | Sustainability Benefits |
|---|---|---|---|
| Single-Atom Catalysts (SACs) | Maximum atom efficiency, high activity, and superior selectivity [67]. | Biomass conversion, CO₂ reduction, renewable energy processes [67] [68]. | Minimizes use of precious metals; enhances selectivity to reduce waste. |
| Metal-Organic Frameworks (MOFs) | Ultra-high surface area, tunable porosity, and customizable functionality [67] [68]. | Biomass conversion, biodiesel production, gas storage [68]. | High activity allows for milder reaction conditions; designable for specific reactions. |
| Non-Precious Metal Catalysts | Based on earth-abundant elements like Ni, Co, Fe, and Cu [68]. | Water splitting for hydrogen production, replacement for rare-earth magnets [52] [68]. | Reduces reliance on scarce, expensive, and geopolitically concentrated resources. |
| Enzymes (Biocatalysts) | Exceptional selectivity, operate in water at ambient temperature and pressure [63]. | Pharmaceutical synthesis, biofuel production, fine chemicals [63]. | Dramatically reduces energy consumption and organic solvent use; biodegradable. |
| Ionic Liquids (ILs) & Deep Eutectic Solvents (DES) | Low volatility, tunable properties, can act as catalysts and solvents [67] [69]. | Solvent-free synthesis, metal extraction from e-waste, biomass processing [52] [69]. | Enables solvent-free conditions or replaces volatile organic compounds (VOCs); recyclable. |
Innovative Process Technologies
Beyond new materials, novel engineering approaches are revolutionizing how chemical reactions are performed.
Mechanochemistry
This solvent-free technique uses mechanical energy (e.g., from ball milling) to drive chemical reactions [52]. It eliminates the environmental and safety issues associated with solvents, which often account for the majority of waste in pharmaceutical and fine chemical production. Mechanochemistry enables reactions involving low-solubility reactants and is being scaled up for industrial application in pharmaceuticals and materials science [52].
In-Water and On-Water Catalysis
Replacing toxic organic solvents with water is a major goal of green chemistry. Contrary to traditional assumptions, many reactions can be accelerated in or on water, leveraging its unique hydrogen bonding and interface properties [52]. This approach reduces production costs, toxicity, and flammability risks, and is seeing wider adoption in pharmaceutical R&D [52].
Continuous Flow Chemistry
Transitioning from traditional batch processes to continuous flow represents a systems-level improvement in chemical manufacturing [62]. Flow reactors offer superior heat and mass transfer, allowing for more precise control over reaction conditions, enhanced safety, and easier scalability. This often leads to improved yields and selectivity while reducing energy consumption and waste.
AI-Guided Reaction Optimization
Artificial intelligence is transforming catalyst and reaction design. AI tools can predict reaction outcomes, optimize conditions for sustainability metrics (atom economy, energy efficiency, toxicity), and suggest safer synthetic pathways [52]. This reduces reliance on trial-and-error experimentation, accelerating the development of greener processes. AI can also predict catalyst behavior without physical testing, reducing waste and energy use in the research phase [52].
Experimental Protocols for Catalytic Transformations
Objective: To form a biaryl compound via a palladium-catalyzed carbon-carbon bond formation using solvent-free mechanochemical conditions.
Principle: The Suzuki-Miyaura coupling is a quintessential atom-economical reaction that joins an aryl halide and an organoborane. Performing it via ball milling eliminates the need for solvent, enhancing the green credentials of an already efficient process [52] [64].
Materials:
- Aryl halide (e.g., 4-bromotoluene)
- Arylboronic acid (e.g., phenylboronic acid)
- Palladium catalyst (e.g., Pd(PPh₃)₄)
- Base (e.g., K₂CO₃)
- Grinding jars and balls (e.g., stainless steel or zirconia)
Procedure:
- Charge: In an inert atmosphere glovebox, place the aryl halide (1.0 mmol), arylboronic acid (1.2 mmol), palladium catalyst (2 mol %), and base (2.0 mmol) into a grinding jar with grinding balls (e.g., two 10 mm balls).
- Mill: Secure the jar in a high-energy ball mill and process at 500 rpm for 60 minutes.
- Work-up: After milling, open the jar and add a small amount of ethyl acetate (5-10 mL) to dissolve the product. Filter the solution to remove the solid base and catalyst residues.
- Purification: Concentrate the filtrate under reduced pressure. Purify the crude product by flash chromatography or recrystallization to obtain the pure biaryl product.
Key Advantages:
- Solvent Elimination: Avoids the use of large volumes of toxic organic solvents typically used in Suzuki reactions.
- High Efficiency: Excellent yield and selectivity can be achieved in a short reaction time.
- Simple Setup: The procedure is straightforward and avoids complex heating or stirring setups.
Objective: To synthesize a key chiral intermediate for an API (e.g., Edoxaban) using an immobilized lipase in water.
Principle: Enzymes provide unmatched selectivity under mild, aqueous conditions. This protocol highlights the dramatic reductions in solvent use and process complexity achievable with biocatalysis.
Materials:
- Substrates (e.g., prochiral ester or amine)
- Immobilized Lipase (e.g., Candida antarctica Lipase B on acrylic resin)
- Aqueous Buffer (e.g., phosphate buffer, pH 7.0)
- Glass reactor with temperature control and mechanical stirring
Procedure:
- Reaction Setup: Charge the aqueous buffer (the only solvent) and substrates into the reactor. Add the immobilized lipase (10-20% by weight of substrates).
- Reaction: Stir the mixture gently at 25-30°C, monitoring reaction progress by TLC or HPLC.
- Filtration: Upon completion, filter the reaction mixture to recover the solid, reusable immobilized enzyme.
- Extraction: Extract the aqueous filtrate with a minimal amount of a biodegradable solvent (e.g., ethyl acetate) to isolate the product.
- Purification: Concentrate the organic phase to obtain the product, which may be pure enough to proceed to the next step without further purification.
Key Advantages:
- Green Solvents: Uses water as the primary reaction medium, reducing organic solvent use by up to 90% [63].
- Mild Conditions: Proceeds at room temperature and neutral pH, drastically cutting energy consumption.
- Catalyst Reusability: The immobilized enzyme can be filtered, washed, and reused multiple times.
- High Selectivity: Eliminates the need for protecting groups and complex purification, improving atom economy.
The Scientist's Toolkit: Essential Reagents and Materials
Table 3: Research Reagent Solutions for Energy-Efficient Catalysis
| Reagent/Material | Function | Green Chemistry Rationale |
|---|---|---|
| Palladium Catalysts (e.g., Pd(PPh₃)₄) | Facilitates cross-coupling reactions for C-C and C-X bond formation [66]. | Enables highly atom-economical transformations like the Suzuki reaction, replacing multi-step stoichiometric routes [64]. |
| Immobilized Lipases (e.g., CAL-B) | Biocatalyst for enantioselective hydrolyses, transesterifications, and amide formations [63]. | Operates in water at ambient temperatures, is biodegradable, and can be reused, reducing E-factor [63]. |
| Deep Eutectic Solvents (DES) | Serve as recyclable, biodegradable reaction media or catalysts [52]. | Replace volatile organic compounds (VOCs); can be synthesized from non-toxic, renewable precursors (e.g., choline chloride and urea) [52]. |
| Earth-Abundant Metal Salts (e.g., FeCl₃, Cu(OTf)₂) | Lewis acid or transition-metal catalyst for various transformations, including C-H functionalization [66] [69]. | Lower cost and environmental footprint compared to precious metals like Pt, Pd, or Ru; reduce resource criticality [52] [68]. |
| Polyethylene Glycol (PEG) | A recyclable, non-toxic polymer that can act as a solvent and catalyst support [69]. | Provides a solvent-free alternative for homogeneous catalysis; facilitates catalyst recovery and reuse, minimizing waste [69]. |
Visualization of Catalytic System Workflows
Workflow for Developing an Energy-Efficient Catalytic Process
Diagram 1: Sustainable Catalysis Development Workflow
Energy Profile of Catalytic vs. Stoichiometric Reactions
Diagram 2: Catalytic Reaction Energy Profile
The integration of catalysis as a core design element is indispensable for advancing energy efficiency and atom economy in chemical processes. The historical trajectory of the sustainable chemistry movement shows a clear evolution from pollution cleanup to prevention, and now toward a holistic, systems-based approach [62]. The future of catalysis lies in the continued development of smart catalytic systems—such as single-atom catalysts, highly selective enzymes, and recyclable homogeneous catalysts—coupled with innovative process technologies like mechanochemistry and continuous flow [52] [67] [69].
The adoption of artificial intelligence and machine learning will further accelerate the discovery and optimization of these systems, enabling predictive design of catalysts and reactions that prioritize sustainability metrics from the outset [52]. Furthermore, the principles of circular chemistry will drive the application of catalysis to waste valorization, using designed catalysts like deep eutectic solvents to recover critical materials from end-of-life products and create a truly circular economy for chemicals [52] [61]. As the industrial catalyst market shifts toward these efficiency and sustainability goals, the synergy between novel catalytic materials, engineered reactor systems, and digital tools will define the next chapter of green and sustainable chemistry [65].
Navigating the Green Shift: Overcoming Barriers and Optimizing for Economic and Regulatory Success
The sustainable chemistry movement represents a paradigm shift from traditional pollution control and risk management toward the proactive design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances [54]. This transition, evolving since the 1990s with the formalization of green chemistry principles, now confronts the critical implementation phase where theoretical advantages meet practical deployment challenges [70] [54]. For researchers, scientists, and drug development professionals, the adoption of green technologies stands at the intersection of pressing global sustainability imperatives and rigorous scientific and economic realities. The movement has progressed from conceptual frameworks to legislative reality with the 2021 Sustainable Chemistry Research and Development Act in the United States, signaling a maturation of the field that now demands addressing fundamental adoption barriers [54].
This technical guide examines the three interconnected hurdles that consistently impede widespread green process adoption: cost constraints spanning research to commercialization, scalability limitations from laboratory validation to industrial implementation, and technical feasibility challenges involving performance and integration. Understanding these constraints within their historical context provides a framework for developing strategic solutions that can accelerate the integration of sustainable chemistry principles across research and industrial sectors, particularly in pharmaceutical development where process efficiency and environmental responsibility are increasingly aligned.
Theoretical Framework: Understanding Adoption Drivers and Barriers
The adoption of green technologies can be understood through integrated behavioral and systemic theoretical lenses. The Theory of Planned Behavior (TPB) and Diffusion of Innovations Theory (DIT) provide complementary frameworks for analyzing adoption dynamics [71]. TPB emphasizes how individual attitudes, subjective norms, and perceived behavioral control influence behavioral intentions toward green technology adoption, while DIT focuses on how innovations spread through social systems based on characteristics like relative advantage, compatibility, and observability [71].
For green technologies, this integration reveals a critical insight: cognitive processes and individual decision-making are shaped by systemic enablers such as government support and policy frameworks [71]. This theoretical foundation helps explain why technically sound green chemistry solutions often face adoption resistance despite their environmental advantages. The perceived economic burden of ecological policies often creates ideological divides that hinder implementation, necessitating approaches that balance ecological performance with economic profit [71].
Complexity Theory in Technology Adoption
Beyond behavioral frameworks, complexity theory provides crucial insights into green technology adoption dynamics. When firms choose between clean (green) and conventional (brown) technologies, the system exhibits increasing returns to adoption and path dependence [72]. Small historical accidents can have large, unpredictable long-term effects on adoption patterns, creating lock-in effects for established brown technologies despite the clear environmental advantages of green alternatives [72].
This complex adaptive systems perspective explains why optimal Pigouvian taxes (taxes on activities with negative externalities) and long-run green technology adoption might sometimes be in conflict, and why the optimal temporal pattern of subsidies is typically decreasing over time [72]. The unpredictable emergent properties of these systems underscore the importance of strategic public policy interventions to navigate the stochastic dynamics that result in bifurcations and tipping points in technology adoption [72].
Historical Context of Sustainable Chemistry Movement
The sustainable chemistry movement emerged from a growing recognition in the 1980s that chemicals management strategy needed to shift away from treating and controlling chemical hazards and waste toward avoiding environmental and health impacts at their source [54]. This culminated in the 1990 Pollution Prevention Act, which established prevention as a national priority. The field was further solidified in the mid-1990s with the EPA's Design for Environment program, the Presidential Green Chemistry Challenge Awards, and the 1998 publication of Paul Anastas and John Warner's seminal work, Green Chemistry: Theory and Practice [54].
Despite more than two decades of development, green and sustainable chemistry remains niche in both academic chemistry and the chemical industry [54]. Barriers include a lack of policy incentives and coordinated government leadership, limited funding for targeted research, few incentives for educators to teach green chemistry, and the entrenched economic advantage of existing chemicals and processes that utilize capitalized technologies and are tightly integrated into global supply chains [54]. The World Health Organization estimates that health damage from chemical exposures results in more than 1.6 million lives and 45 million disability-adjusted life-years lost globally each year, costing up to 10% of global GDP, providing a compelling rationale for overcoming these adoption barriers [54].
Table 1: Historical Evolution of Sustainable Chemistry Policy and Implementation
| Time Period | Key Developments | Implementation Focus |
|---|---|---|
| 1960s-1980s | Rachel Carson's documentation of DDT impacts; Love Canal, Bhopal, and ozone hole incidents; Passage of TSCA, RCRA, Superfund | Regulatory compliance, pollution control, risk management |
| 1990s | Pollution Prevention Act (1990); EPA Design for Environment program; Green Chemistry Challenge Awards; Anastas & Warner's Green Chemistry: Theory and Practice (1998) | Pollution prevention, source reduction, green chemistry principles |
| 2000-2010 | First Green Chemistry R&D Act introduced (2004); Early industry adoption in pharmaceutical sector; America COMPETES Act reauthorization | Research and development, early commercialization, educational initiatives |
| 2011-Present | Sustainable Chemistry R&D Act passage (2021); GAO report on sustainable chemistry; International Nobel Declaration on Green Chemistry (2025) | Coordinated federal strategy, public-private partnerships, circular economy integration |
Quantitative Analysis of Adoption Hurdles
Cost-Related Barriers
Cost considerations present multifaceted barriers to green process adoption, encompassing high initial capital investment, production cost disparities, and financial uncertainty. Recent empirical studies utilizing explainable artificial intelligence (XAI) approaches have quantified how economic factors influence green technology adoption decisions [73]. For instance, in the German context, adoption of battery electric vehicles was strongly associated with income levels, highlighting the economic accessibility barrier [73].
The chemical industry faces particular cost challenges, with sustainable chemistry solutions often competing against established processes that benefit from depreciated infrastructure and optimized supply chains [53]. The International Energy Agency notes that green hydrogen production costs remain 2-3 times higher than conventional production methods, creating a significant economic barrier despite environmental advantages [74]. Additionally, complex technology adoption environments characterized by increasing returns to scale and heterogeneous firm capabilities create economic conditions where small initial advantages for conventional technologies can lead to long-term lock-in effects [72].
Table 2: Cost Analysis of Selected Green Technologies vs. Conventional Alternatives
| Technology | Green Alternative Cost | Conventional Technology Cost | Key Cost Drivers | Regulatory Incentives |
|---|---|---|---|---|
| Green Hydrogen Production | $4-6/kg [74] | $1.50-2.50/kg (gray hydrogen) | Electrolyzer efficiency, renewable electricity prices, storage infrastructure | Investment tax credits, production tax credits, R&D grants |
| Sustainable Aviation Fuel (SAF) | 2-4x conventional jet fuel [74] | Baseline | Feedstock availability, conversion efficiency, scaling limitations | Blending mandates, carbon credits, low-carbon fuel standards |
| Advanced Biofuels | $3.50-6.50/gallon gasoline equivalent | $2.00-3.00/gallon | Biomass preprocessing, conversion yields, catalyst development | Renewable Fuel Standard, California LCFS, investment subsidies |
| CO2 to Chemicals | $500-900/ton product | $300-600/ton (fossil-based) | Capture energy, catalyst lifetime, separation costs | Carbon credit markets, 45Q tax credits, procurement preferences |
Scalability Challenges
Scalability represents a critical transition point from laboratory success to industrial impact. The "valley of death" between pilot-scale demonstration and full commercial implementation remains particularly formidable for green chemistry innovations [53]. Scalability challenges manifest across multiple dimensions:
Technical Scalability involves maintaining process efficiency, selectivity, and control when moving from gram to ton scale. For example, electrochemical processes that demonstrate excellent performance in laboratory cells often face mass transport limitations and heating management challenges at commercial scale [74]. Similarly, advanced biofuel production encounters feedstock consistency variability and preprocessing bottlenecks when scaling from bench to continuous operation [74].
Infrastructure Scalability requires compatible manufacturing, distribution, and end-use systems. The adoption of battery electric vehicles depends not only on vehicle technology but also on charging network density and grid capacity [73]. For green hydrogen, scaling faces storage safety concerns and transportation logistics that require substantial infrastructure investment [74].
Supply Chain Scalability ensures reliable access to sustainable feedstocks. The transition from fossil-based to bio-based chemical production requires developing entirely new agricultural networks, collection systems, and preprocessing facilities [53]. The pharmaceutical industry faces particular challenges in securing consistent quality bio-based solvents and renewable specialty chemicals at commercial quantities [54].
Technical Feasibility Limitations
Technical feasibility hurdles encompass both performance specifications and integration compatibility with existing manufacturing systems. Explainable AI models analyzing regional differences in technology adoption reveal that technical factors like solar radiation levels significantly influence photovoltaic adoption, while dwelling size affects the feasibility of residential energy technologies [73].
Performance gaps persist in several green technology domains. Current sustainable aviation fuels (SAF) face challenges with energy density and compatibility with existing aircraft systems [74]. CO2 conversion technologies struggle with catalyst lifetime and product separation efficiency [74]. In the pharmaceutical sector, green chemistry alternatives must meet stringent purity requirements and regulatory validation standards while maintaining economic viability [54].
System integration challenges emerge when introducing green processes into existing manufacturing infrastructure. Intermittent renewable energy integration requires sophisticated process control strategies for electrochemical manufacturing [74]. Circular economy implementation faces technical hurdles in material identification, separation efficiency, and quality consistency for recycled feedstocks [53].
Diagram 1: Interrelationship of Green Technology Adoption Barriers and Intervention Strategies. The diagram illustrates how cost, scalability, and technical feasibility hurdles interact and potential policy and research intervention points.
Methodologies for Assessing and Overcoming Adoption Hurdles
Experimental Protocols for Feasibility Assessment
Accelerated Lifecycle Testing for Green Technologies Purpose: Evaluate long-term performance and degradation patterns of green technologies under accelerated conditions to predict operational lifespan and identify failure modes. Methodology:
- Stress Condition Definition: Identify critical operational parameters (temperature, pressure, pH, cycling frequency) that influence material degradation and process efficiency
- Accelerated Testing Protocol: Subject technologies to controlled stress conditions (e.g., thermal cycling, load variation, chemical exposure) while monitoring performance metrics
- Degradation Modeling: Apply Arrhenius-based models or power law relationships to extrapolate accelerated results to normal operating conditions
- Failure Analysis: Conduct post-testing materials characterization (SEM, XRD, FTIR) to identify degradation mechanisms and inform redesign
Techno-Economic Analysis (TEA) Framework Purpose: Systematically evaluate economic viability and identify cost drivers across technology development stages. Methodology:
- Process Modeling: Develop detailed process flow diagrams with mass and energy balances for both laboratory and scaled-up configurations
- Capital Cost Estimation: Use factorial estimation methods (equipment factoring) or detailed item-by-item costing for major equipment
- Operating Cost Assessment: Calculate variable costs (feedstocks, utilities, catalysts) and fixed costs (labor, maintenance, overhead)
- Financial Modeling: Apply discounted cash flow analysis with appropriate risk-adjusted discount rates
- Sensitivity Analysis: Identify critical cost drivers and breakeven points for key performance parameters
Cross-Scale Integration Protocol Purpose: Evaluate compatibility and performance when integrating green technologies with existing industrial infrastructure. Methodology:
- Interface Mapping: Identify all physical, energy, and information interfaces between new technology and existing systems
- Compatibility Testing: Assess material compatibilities, control system integration, and safety system interactions
- Performance Benchmarking: Compare integrated system performance against conventional alternatives using key performance indicators (KPIs)
- Transition Planning: Develop phased implementation strategies to minimize disruption during technology transition
Digital Tools for Adoption Analysis
Digital technologies provide powerful methodologies for analyzing and optimizing green technology adoption. Explainable Artificial Intelligence (XAI) models can identify key influencing factors for technology adoption by analyzing demographic, geographic, political, and socio-economic features [73]. These machine learning approaches complement traditional diffusion research by focusing on spatial aspects and actual adoption decisions rather than just intentions and temporal dynamics [73].
Digital dynamic capabilities (DDC) enable organizations to integrate, develop, and adjust internal and external digital competencies to respond to changing environments [75]. In sustainability contexts, DDC enhances sustainable performance by improving operational efficiency through advanced digital tools like big data analytics, IoT devices, and artificial intelligence [75]. These technologies allow organizations to monitor and optimize resource utilization, reduce waste, and enhance energy efficiency.
Table 3: Digital Tools for Green Technology Implementation and Optimization
| Digital Tool | Primary Function | Application Example | Implementation Requirements |
|---|---|---|---|
| Explainable AI (XAI) | Identify key adoption drivers using SHapley Additive exPlanations | Regional analysis of PV and EV adoption factors [73] | Demographic, geographic, political, and socio-economic datasets |
| Digital Twins | Virtual replication of physical assets for process optimization | Testing process modifications before implementation [53] | IoT sensors, real-time data integration, computational modeling capability |
| AI-Driven Analytics | Predictive modeling of emission reduction scenarios | Decarbonization strategy optimization for industrial processes [74] | Historical operational data, process knowledge, computing infrastructure |
| Blockchain Systems | Supply chain transparency and material traceability | Tracking sustainable feedstocks and circular economy flows [53] | Standardized data protocols, participant integration, verification mechanisms |
Diagram 2: Integrated Methodology Framework for Green Technology Assessment. The diagram shows how experimental protocols and digital tools combine to address different aspects of feasibility assessment.
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Research Materials and Analytical Tools for Green Technology Development
| Reagent/Technology | Function | Application Context | Sustainability Consideration |
|---|---|---|---|
| Ionic Liquids | Green solvents with tunable properties | Replacement for volatile organic compounds in separation processes | Low vapor pressure reduces atmospheric emissions; potential biodegradability concerns |
| Heterogeneous Catalysts | Reusable solid-phase catalysts | Esterification, transesterification, and other synthesis reactions | Eliminates homogeneous catalyst waste; enables continuous flow processes |
| Bio-Based Feedstocks | Renewable carbon sources | Chemical synthesis from agricultural waste, algal oils, forestry residues | Reduces fossil resource depletion; potential land use competition issues |
| Electrochemical Cells | Electron-mediated transformations | CO2 conversion, hydrogen production, organic electrosynthesis | Utilizes renewable electricity; avoids stoichiometric oxidants/reductants |
| Enzyme Systems | Biocatalysts for specific transformations | Pharmaceutical intermediate synthesis, polymer production | High selectivity reduces waste; mild operating conditions save energy |
| Supercritical Fluids | Tunable solvent media | Extraction, reaction media, cleaning applications | Replaces halogenated solvents; typically uses CO2 from waste streams |
| Nanostructured Materials | High-surface-area functional materials | Catalysis, adsorption, energy storage | Enhanced efficiency reduces material requirements; potential toxicity concerns |
| Flow Reactors | Continuous process intensification | Chemical synthesis with improved heat and mass transfer | Reduced reactor volume, improved safety, better energy integration |
The adoption of green processes continues to face significant hurdles related to cost, scalability, and technical feasibility, but integrated strategies drawing from theoretical frameworks and empirical evidence provide pathways forward. The historical context of the sustainable chemistry movement reveals a gradual transition from pollution control to preventive design, now supported by legislative frameworks like the Sustainable Chemistry Research and Development Act [54].
Future research should focus on several critical directions. First, advanced materials development for catalysts, membranes, and electrodes can address performance gaps while reducing cost premiums. Second, process intensification strategies that integrate reaction and separation operations can improve energy efficiency and reduce capital costs. Third, digital integration through AI, machine learning, and IoT technologies can optimize system performance and enable predictive maintenance [75]. Finally, circular economy business models that create value from waste streams can improve economic viability while reducing environmental impact.
For the pharmaceutical industry specifically, priorities include developing green chemistry metrics tailored to drug development, establishing continuous manufacturing platforms that enhance efficiency, and creating standardized assessment protocols for evaluating green technology alternatives. By addressing these challenges through collaborative research, policy support, and industrial implementation, the sustainable chemistry movement can transition from niche applications to mainstream adoption, ultimately achieving its goal of chemical products and processes that reduce or eliminate hazards to human health and the environment.
The movement toward sustainable chemistry represents a fundamental shift in how society designs, manufactures, and regulates chemical products. This paradigm responds to the historical legacy of environmental contamination and health impacts from persistent, bioaccumulative, and toxic substances. Among these, per- and polyfluoroalkyl substances (PFAS) have emerged as a primary concern due to their extreme environmental persistence and documented health risks [76]. The sustainable chemistry framework mandates moving beyond mere functional replacement to a holistic assessment that considers entire chemical lifecycles, thereby avoiding "regrettable substitutions" where one hazardous chemical is replaced by another with similar problematic properties [77] [76]. This whitepaper provides technical guidance for researchers and development professionals seeking to replace PFAS and other persistent chemicals with truly sustainable alternatives, employing rigorous assessment protocols and emerging technologies.
Historical Context and Regulatory Landscape
The PFAS Problem
PFAS comprise a large group of synthetic fluorinated organic chemicals valued for their exceptional stability and surface-tension-lowering properties [76]. Their molecular structure, featuring strong carbon-fluorine bonds, confers both valuable functional properties and extreme environmental persistence, earning them the designation "forever chemicals" [76]. Historically deployed in numerous industrial and consumer applications including fire-fighting foams, non-stick coatings, and water-repellent textiles, PFAS now contaminate global ecosystems [77]. Research has linked certain PFAS compounds to multiple adverse health effects in humans, including developmental issues, liver damage, and immune system suppression [77] [76].
The Regulatory Response
Intensifying scientific understanding of PFAS risks has triggered a global regulatory response. In the United States, the Environmental Protection Agency (EPA) has initiated multiple rulemakings targeting these substances, demonstrating an evolving regulatory approach. Key recent developments include:
- CERCLA Hazardous Substance Designations: The EPA has committed to retaining the designation of PFOA and PFOS as hazardous substances under the Comprehensive Environmental Response, Compensation, and Liability Act (Superfund), signaling continued focus on cleanup and liability for these specific compounds [78] [79].
- Drinking Water Regulations: The EPA is narrowing its initial drinking water rule by maintaining Maximum Contaminant Levels (MCLs) for PFOA and PFOS while withdrawing standards for other PFAS compounds (PFHxS, PFNA, HFPO-DA) and their mixtures [78] [79].
- Multi-Statutory Approach: The September 2025 Unified Regulatory Agenda outlines upcoming actions across multiple environmental statutes, including the Clean Water Act (CWA), Resource Conservation and Recovery Act (RCRA), and Toxic Substances Control Act (TSCA) [79]. These will impose new monitoring, reporting, and corrective action requirements.
Table 1: Key U.S. Regulatory Developments for PFAS (as of October 2025)
| Regulatory Area | Statute | Action | Timeline | Potential Impact |
|---|---|---|---|---|
| Hazardous Substance Listing | CERCLA | Retaining PFOA/PFOS as hazardous substances; developing framework for future designations | Framework expected 2025-2026 | Increases liability for releases; mandates cleanup |
| Drinking Water | SDWA | Finalizing rule to narrow scope to only PFOA/PFOS; extending compliance deadlines | Proposed rule October 2025 | Reduces monitoring burden for water systems |
| Waste Management | RCRA | Finalizing listing of nine PFAS as hazardous constituents | Final rule April 2026 | Requires corrective action for releases at permitted facilities |
| Industrial Discharges | CWA | Proposing new PFAS monitoring in NPDES permits; developing Effluent Limitations Guidelines | Proposed rules Late 2025/Early 2026 | Imposes new discharge limits and monitoring for manufacturers |
| Chemical Reporting | TSCA | Proposing modifications to PFAS reporting rule, potentially creating exemptions | Proposed rule December 2025 | May reduce reporting obligations for certain importers |
This regulatory momentum, combined with similar actions in the European Union under the REACH restriction proposal [76], creates a powerful business and ethical case for the proactive development and adoption of safer alternatives.
A Framework for Alternative Assessment
The Functional Substitution Approach
To avoid regrettable substitution, a systematic methodology is required. The functional substitution approach moves beyond identifying "drop-in" chemical replacements to fundamentally re-evaluating the needed function within a specific application [76]. This framework involves three levels of analysis:
- Chemical Function: The specific technical function provided by the substance (e.g., reducing surface tension, providing thermal stability).
- End-Use Function: The property the substance imparts to the final product or process (e.g., stain resistance in textiles, non-stick properties in cookware).
- Function as a Service: The ultimate benefit provided to the user or society (e.g., convenient food release, protection from liquid spills).
This hierarchical analysis opens the solution space to include alternative materials, product redesigns, and entirely new technologies, rather than being limited to alternative chemical substances [76].
Defining Alternatives and Assessment Criteria
In this context, an "alternative" is defined as any means to provide a function comparable to PFAS. The typology of alternatives includes [76]:
- Alternative Substances/Materials: Different chemicals or materials that provide similar functions (e.g., graphene oxide coatings, silicone-based polymers).
- Alternative Products/Processes: Changes in formulation or design that eliminate the need for the function altogether.
- Alternative Technologies: Entirely different systems that deliver the same service.
A robust assessment should evaluate alternatives based on the following criteria:
- Hazard Profile: Toxicity, ecotoxicity, and other inherent hazards.
- Environmental Fate: Persistence, bioaccumulation potential, and mobility in different environmental media.
- Performance: Efficacy in delivering the required function under real-world conditions.
- Economic Viability: Cost relative to incumbent and other alternatives.
- Technical Maturity: Readiness for commercial deployment.
Diagram 1: Functional substitution workflow
Emerging Alternatives and Replacement Technologies
Promising Substitute Materials
Research and commercial development have identified numerous promising pathways for replacing PFAS across different application sectors. A comprehensive 2025 study identified 530 PFAS-free alternatives across 325 different applications, with potentially suitable alternatives available for 40 applications, though 83 applications still lack identified alternatives [76]. Key innovations include:
- Graphene Oxide-Based Materials: Researchers at Northwestern University have developed a graphene oxide-based material that provides exceptional water- and oil-resistance for paper-based food packaging [80]. This material is non-toxic, enhances substrate strength by 30-50%, and is compostable and recyclable, addressing the function as a service (effective packaging) without the persistence hazard of PFAS [80].
- Organosilicon Compounds: Silicon-based chemistry offers a class of alternatives for water- and stain-repellent applications. While a full lifecycle assessment is always required, these compounds generally present a different environmental profile than fluorinated chemicals.
- Natural-Based Compounds and Derivatives: Materials derived from cellulose, starch, and other biopolymers are being engineered to provide barrier properties, particularly in food serviceware and packaging [76].
The Risk of "Regrettable Substitution"
The transition away from legacy PFAS must be undertaken cautiously, as some early alternatives have themselves raised environmental health concerns. Compounds such as HFPO-DA (GenX), ADONA, 6:2 Cl-PFAES, and 6:2 FTAB have seen increased use as PFOA and PFOS are phased out [77]. However, studies show these alternatives can also exhibit long-range transport, environmental persistence, and multi-dimensional toxicity to biological cells and organ functions [77]. This underscores the critical importance of thorough pre-market testing that evaluates not just acute toxicity but also long-term environmental fate and chronic health impacts.
Table 2: Assessment of Representative PFAS Alternatives
| Alternative Name/Type | Primary Applications | Key Advantages | Potential Concerns / R&D Needs |
|---|---|---|---|
| Graphene Oxide | Food packaging, disposable tableware, cardboard | Non-toxic, compostable, enhances strength, affordable | Scaling production, FDA food-contact approval, long-term biodegradability studies [80] |
| Organosilicon Compounds | Textiles, coatings, cosmetics | Different chemistry from PFAS, established manufacturing | Requires full lifecycle assessment of degradation products [76] |
| Natural-Based Polymers (e.g., cellulose derivatives) | Food packaging, consumer goods | Biobased, often biodegradable, renewable sourcing | Barrier performance under diverse conditions, cost competitiveness [76] |
| Emerging Fluorinated Alternatives (e.g., HFPO-DA, ADONA) | Industrial applications, polymer production | "Short-chain" or polymerized chemistry | Evidence of persistence, mobility in water, and toxicity; risk of regrettable substitution [77] |
Experimental Protocols for Evaluating Alternatives
Standardized Testing Workflow
A rigorous, multi-phase testing protocol is essential for comprehensively evaluating potential PFAS alternatives. The following workflow provides a structured methodology for assessment.
Diagram 2: Alternative assessment workflow
Detailed Methodologies for Key Assays
Phase I: In Silico Screening
- Toxicity Prediction: Utilize Quantitative Structure-Activity Relationship (QSAR) models and read-across methodologies with existing databases to predict potential endocrine disruption, mutagenicity, and other toxicological endpoints.
- Persistence & Bioaccumulation Prediction: Employ predictive software to estimate degradation half-lives in air, water, and soil, as well as bioaccumulation factors (BAF) and bioconcentration factors (BCF). Prioritize alternatives predicted to be readily biodegradable (e.g., >70% degradation in 28-day test) with low bioaccumulation potential (Log Kow < 3.5).
Phase II: In Vitro Toxicological Assessment
- Cytotoxicity Assays: Expose mammalian cell lines (e.g., HepG2 liver cells) to a range of alternative concentrations (0.1-100 µg/mL) for 24-72 hours. Assess cell viability using standardized MTT or Alamar Blue assays. Calculate IC50 values and compare to reference PFAS.
- High-Throughput Toxicological Screening: Utilize ToxCast or similar platforms to screen for activity across hundreds of cellular signaling pathways, including nuclear receptor activation and stress response pathways.
Phase III: Application-Specific Performance Testing
- Barrier Property Evaluation (for coatings): Apply alternative to standard substrate (e.g., WHATMAN #1 filter paper) at multiple coating weights. Quantify performance via:
- Water Holdout: Cobb test (TAPPI T441) at 1-minute and 10-minute intervals.
- Oil/Grease Resistance: Kit test (TAPPI T507) using solutions of castor oil, toluene, heptane, and hexane.
- Repellency Durability: Subject samples to abrasion (Taber Abraser) and flexing (MIT Folding Endurance) before re-testing.
Phase IV: Environmental Fate Studies
- Aerobic Aquatic Degradation: Conduct OECD 301 or ISO 9439 tests to determine ultimate biodegradation. Measure dissolved organic carbon removal over 28 days; >60% removal indicates ready biodegradability.
- Bioaccumulation Assay: Perform fish accumulation tests (OECD 305) with common species like Cyprinodon variegatus. Determine steady-state BCF; values <100 indicate low bioaccumulation potential.
The Scientist's Toolkit: Research Reagents and Materials
Table 3: Essential Reagents for PFAS Alternative Research
| Reagent/Material | Function in Research | Application Context |
|---|---|---|
| Graphene Oxide Dispersion | Formulation of water-based barrier coatings | Sustainable packaging, replacement for PFAS in food serviceware [80] |
| Organosilane Precursors | Synthesis of silicon-based repellent polymers | Textile finishing, surface coatings as PFAS alternative [76] |
| Modified Cellulose (e.g., TEMPO-oxidized) | Biobased material for barrier formation | Compostable packaging, bio-based alternatives to PFAS-treated paper [76] |
| Standard Reference PFAS (PFOA, PFOS) | Positive controls for performance and toxicity comparison | Baseline establishment in experimental studies |
| Cytotoxicity Assay Kits (e.g., MTT, Alamar Blue) | Assessment of cellular toxicity in mammalian cell lines | High-throughput screening of alternative safety |
| QSAR Software Tools | Predictive toxicology and environmental fate modeling | Early-stage screening and prioritization of candidate molecules |
The transition from PFAS and other persistent chemicals represents both a significant challenge and a profound opportunity to advance the principles of sustainable chemistry. Success requires a multidisciplinary approach that integrates materials science, toxicology, environmental chemistry, and regulatory strategy. The framework outlined in this whitepaper—centered on functional substitution, rigorous assessment protocols, and learning from past substitutions—provides a roadmap for researchers and product developers. While significant progress has been made, with market-ready alternatives available for some applications, critical research gaps remain for approximately 25% of known PFAS uses where no alternatives have yet been identified [76]. The continued collaboration between academia, industry, and regulators, supported by intelligent screening tools and a commitment to transparent assessment, will accelerate the development of innovations that deliver necessary function without perpetuating harm, fulfilling the enduring promise of the sustainable chemistry movement.
The concept of a circular economy represents a fundamental transformation in industrial systems, challenging the traditional linear "take-make-dispose" model by creating closed-loop systems where resources maintain maximum value throughout their lifecycle [81]. This paradigm shift finds its roots in the sustainable chemistry movement, which emerged from growing recognition that traditional chemical processes and material designs were generating unsustainable waste streams and environmental impacts. The recent "Chemistry for the Future" Nobel Declaration underscores this connection, stating that "sustainability without innovation is impossible and innovation without sustainability would be ruinous" [70]. This declaration, signed by global scientific leaders, emphasizes that sustainable chemistry must integrate the goal of reducing or eliminating harm to people and the planet by design—principles that form the very foundation of circular economy integration in manufacturing.
Within manufacturing industries, circular economy practice has emerged as a potential solution to ongoing global environmental and economic issues [82]. The manufacturing sector faces increasing pressure from regulatory frameworks, resource constraints, and evolving consumer preferences toward sustainable products. Research indicates that businesses implementing circular strategies achieve up to 67% cost savings while reducing environmental impact by 72%, demonstrating the compelling business case for adoption [81]. This technical guide provides researchers, scientists, and manufacturing professionals with comprehensive methodologies for designing systems that prioritize resource recovery and waste valorization, positioning these practices within the broader historical context of sustainable chemistry movement research.
Current Landscape and Market Dynamics
The circular economy market has experienced accelerated adoption, with the global market projected to grow from $656.23 billion in 2024 to $2,659.39 billion by 2035, representing a compound annual growth rate (CAGR) of 13.57% [83]. Manufacturing, consumer goods, and technology sectors demonstrate the highest circular economy implementation rates, with 64% of Fortune 500 manufacturers now incorporating circular principles into core operations [81]. European markets lead adoption rates at 38%, followed by APAC regions at 27% and North America at 19%, creating distinct opportunities for businesses operating across different markets [81].
Table 1: Global Circular Economy Market Projections (2024-2035)
| Year | Market Size (USD Billion) | Year-over-Year Growth | Key Adoption Drivers |
|---|---|---|---|
| 2024 | 656.23 | - | Regulatory pressure, material cost volatility |
| 2027 | 1,125.45* | 13.57% CAGR | EPR policies, consumer demand, brand positioning |
| 2030 | 1,850.72* | 13.57% CAGR | Climate commitments, circular design standardization |
| 2035 | 2,659.39 | 13.57% CAGR | Full regulatory alignment, advanced recycling infrastructure |
*Projected values based on CAGR
Regional variations in adoption reflect differing regulatory approaches and infrastructure development. Europe leads implementation due to sophisticated infrastructure, legal frameworks, and public dedication to environmental sustainability [83]. The European Union's Circular Economy Action Plan has established binding targets requiring 65% municipal waste recycling by 2035 and virtually eliminating landfill disposal for recyclable materials [81]. Extended Producer Responsibility (EPR) regulations now cover 94% of product categories, fundamentally reshaping manufacturer obligations across product lifecycles [81].
The Asia-Pacific region is experiencing the fastest growth, driven by factories adopting circular procedures to meet global demand for environmentally friendly products [83]. Countries like Singapore with closed-loop water management, South Korea with electronic waste management systems, and Japan with longstanding emphasis on material efficiency are leading this transition [83]. China's restrictions on garbage imports have further promoted domestic recycling investments across Asia [83].
Circular Economy Business Models for Manufacturing
Multiple business models have emerged as viable approaches for circular economy implementation in manufacturing, each with distinct implementation frameworks and value propositions.
Product-as-a-Service (PaaS) Models
The Product-as-a-Service model transforms ownership dynamics by providing functional access rather than asset ownership. Philips Lighting's transition to "lighting-as-a-service" demonstrates this model's potential, reducing customer energy costs by 50% while maintaining product ownership and responsibility for maintenance, upgrades, and eventual recycling [81]. Harvard Business Review analysis indicates PaaS models increase customer lifetime value by 37% while reducing manufacturing costs through optimized product design for longevity and modularity [81]. Consumer insights research reveals that 67% of B2B customers prefer service-based models over traditional ownership when total cost of ownership decreases [81].
Remanufacturing and Refurbishment Strategies
Remanufacturing represents a $110 billion global industry, with products achieving 85-95% of original performance at 40-60% of new product costs [81]. Caterpillar's remanufacturing operations have saved customers $8 billion while diverting 2.1 million metric tons of waste from landfills since 2020 [81]. This approach is particularly valuable for manufacturing industries with high-value capital equipment, enabling recovery of embedded energy and materials while offering customers cost-effective alternatives to new equipment.
Industrial Symbiosis and Urban Mining
Industrial symbiosis—where waste outputs from one process become inputs for another—has achieved remarkable results in manufacturing ecosystems. The Kalundborg Symbiosis in Denmark demonstrates this potential, with 30 companies exchanging 3 million tons of materials annually, generating $24 million in collective savings [81]. Urban mining operations now recover valuable materials from waste streams at high purity levels; for example, smartphone recycling recovers over 60 elements, including precious metals like gold, silver, and rare earth elements, with recovered material values exceeding virgin extraction costs by 40% [81].
Assessment Methodologies for Circular Economy Maturity
A pertinent maturity model is required for evaluating and guiding manufacturing organizations toward the implementation of circular practices [82]. Research on manufacturing organizations in developing economies indicates that approximately 51% of studied industries are still in the initial levels of circular transition, having just started to either comprehend the importance or adopt pilot projects to judge the potential of implementing circular practices within their value chain [82].
Table 2: Circular Economy Maturity Assessment Dimensions
| Maturity Dimension | Key Assessment Activities | Relative Importance Weight | Exemplary Manufacturing Practices |
|---|---|---|---|
| Value Creation | R-related activities (reduce, reuse, recycle, recover, etc.) | Highest crucial effect | Product-as-a-service models, waste-to-energy systems |
| Strategic Integration | Executive commitment, circular KPI integration, staff training | High importance | Circular product design mandates, cross-functional circular teams |
| Operations Management | Reverse logistics, collection systems, supplier collaboration | Medium-high importance | Industrial symbiosis networks, take-back programs |
| Technology Enablers | Digital tracking, AI optimization, advanced sorting | Medium importance | Blockchain material tracking, AI-powered predictive maintenance |
| Performance Measurement | LCA implementation, circularity metrics, reporting | Medium importance | Material circularity indicators, environmental product declarations |
The "Value Creation" dimension has been identified as most significant to explaining CE maturity status, in which 'R-related activities' (reduce, reuse, recycle, recover, etc.) exhibit the most crucial effect [82]. Manufacturing organizations at higher maturity levels integrate circular economy practice as a strategic problem in managerial decision-making, leading to improved economic, environmental, and social outcomes [82].
Circular Economy Maturity Pathway
Experimental Protocols for Waste Valorization
Protocol 1: Industrial By-product Valorization in Construction Materials
Objective: Convert industrial waste streams (e.g., phosphogypsum, concrete washing fines) into functional construction materials with verified mechanical properties and reduced environmental impact.
Materials and Equipment:
- Industrial by-products (phosphogypsum, concrete washing fines)
- Water-reducing agents (polycarboxylate-based superplasticizers)
- Foaming agents (protein-based or synthetic)
- Standard mortar mixing equipment
- Compression testing machine
- Microstructure analysis (SEM, XRD)
- Carbon footprint assessment software
Methodology:
- Material Characterization: Conduct physicochemical analysis of industrial by-products to determine composition, particle size distribution, and potential contaminants.
- Mix Design Optimization: Develop optimal formulations using response surface methodology to balance mechanical performance, workability, and environmental impact.
- Curing Process Optimization: Implement dual chemical-physical approaches for curing soluble components (e.g., phosphorus and fluorine in phosphogypsum) to ensure material stability.
- Performance Validation: Test mechanical properties (compressive strength, flexural strength), durability (freeze-thaw resistance, water absorption), and microstructural development.
- Lifecycle Assessment: Quantify carbon footprint reduction and resource efficiency gains compared to conventional materials.
Experimental Findings: Research demonstrates that foamed phosphogypsum with optimized water-reducing agents achieves suitable mechanical performance for non-structural applications, while concrete washing fines incorporation in mortar mixtures reduces carbon footprint by 16-20% while maintaining structural integrity [84].
Protocol 2: Advanced Recycling through Depolymerization
Objective: Implement chemical recycling technologies to break down plastic waste to monomer level for reprocessing into virgin-grade materials.
Materials and Equipment:
- Mixed plastic waste streams (PET, PE, PP)
- Catalysts for depolymerization (zeolites, enzymes)
- Solvent systems for dissolution
- High-pressure/temperature reactor systems
- Purification equipment (distillation, crystallization)
- Analytical instrumentation (GC-MS, FTIR, GPC)
Methodology:
- Feedstock Preparation: Sort, wash, and shred plastic waste to appropriate particle size for processing.
- Solvolysis Process: Subject plastics to chemical breakdown through glycolysis, hydrolysis, or methanolysis at optimized temperature, pressure, and catalyst conditions.
- Monomer Recovery: Separate and purify resulting monomers through distillation, crystallization, or membrane filtration.
- Repolymerization: Convert purified monomers back into polymer resins using conventional polymerization processes.
- Quality Verification: Test recycled polymer properties against virgin material specifications for molecular weight, mechanical properties, and contamination levels.
Technical Considerations: Advanced recycling complements rather than replaces mechanical systems, addressing mixed or contaminated plastics that mechanical recycling cannot handle [85]. Policymakers, including the European Commission, are developing sustainability criteria for chemical recycling outputs to ensure real carbon benefits [85].
Protocol 3: Digital Waste Classification and Sorting
Objective: Implement machine learning algorithms with capacitive sensing for automatic waste classification to improve recycling accuracy and efficiency.
Materials and Equipment:
- Low-cost capacitive sensors
- Microcontroller unit (Arduino, Raspberry Pi)
- Material handling system (conveyor belt, sorting mechanism)
- Machine learning platform (Python with scikit-learn/TensorFlow)
- Waste sample sets (plastics, metals, paper, composites)
Methodology:
- Sensor System Design: Develop capacitive sensing array capable of detecting material dielectric properties.
- Data Acquisition: Collect comprehensive training dataset across multiple waste categories with varying shapes, sizes, and contamination levels.
- Algorithm Development: Train classification models (Support Vector Machines, Random Forest, Neural Networks) using feature extraction from sensor signals.
- System Integration: Combine sensing, classification, and sorting mechanisms into integrated workflow.
- Performance Validation: Test classification accuracy across real-world waste streams and optimize for specific material recovery goals.
Experimental Findings: Research demonstrates that low-cost capacitive sensing systems coupled with machine learning algorithms can achieve high accuracy in automatic waste classification, reducing human intervention and improving recycling outcomes [84].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents and Materials for Circular Economy Experiments
| Reagent/Material | Function in Research | Application Examples | Technical Specifications |
|---|---|---|---|
| Phosphogypsum | Secondary raw material for construction products | Foamed construction materials, soil stabilization | CaSO₄·2H₂O with P₂O₅ and F impurities |
| Concrete Washing Fines | Fine aggregate replacement in mortar/concrete | Cementitious composites, flowable fills | SiO₂, CaCO₃ rich, particle size <100μm |
| Graphite Waste from Acheson Furnaces | Anode material precursor for lithium-ion batteries | Battery manufacturing, energy storage | Carbon content >95%, specific surface area optimization |
| Sewage Sludge and Sawdust | Feedstock for hydrothermal carbonization | Hydrochar production, adsorbent development | Optimal C/N ratio, moisture content adjustment |
| Tea Waste | Catalyst support for environmental remediation | Electro-Fenton process for contaminant removal | Lignocellulosic structure, metal immobilization capability |
| Polyhydroxyalkanoates (PHA) | Bio-based, biodegradable polymer alternative | Compostable packaging, marine-degradable products | Microbial production, tunable thermal properties |
| Mycelium Cultures | Biological binding agent for composite materials | Protective packaging, insulation materials | Rapid growth rate, agricultural waste substrate utilization |
| Supercritical CO₂ | Solvent for waterless processing | Textile dyeing, extraction processes | Pressure >73 bar, temperature >31°C |
Technology Enablers and Digital Innovation
Digital technologies serve as critical enablers for circular economy implementation, with AI, IoT, blockchain, and advanced materials science converging to create previously impossible circular systems [81].
Artificial Intelligence and Predictive Maintenance
AI-powered predictive maintenance extends product lifespans by 40-60% while reducing maintenance costs by 25-30% [81]. Gartner research indicates that IoT sensor networks combined with machine learning algorithms predict component failures with 94% accuracy, enabling just-in-time interventions that maximize asset utilization [81]. Industrial equipment manufacturers implementing AI-driven predictive maintenance report 52% reductions in unplanned downtime and 37% increases in asset availability [81].
Blockchain for Circular Supply Chains
Blockchain technology provides immutable tracking of materials throughout product lifecycles, enabling precise authentication of recycled content, verification of ethical sourcing, and automated execution of circular transactions through smart contracts [81]. Stanford research demonstrates that blockchain-enabled circular supply chains reduce authentication costs by 67% while eliminating 94% of provenance disputes [81]. Material passport systems built on blockchain architecture have achieved 99.7% accuracy in tracking component histories, enabling optimized end-of-life processing that recovers 89% of embedded material value compared to 43% for non-tracked products [81].
Digital-Enabled Circular Manufacturing System
Implementation Framework and ROI Analysis
Demonstrating clear return on investment remains critical for securing executive commitment to circular economy transformation. Comprehensive market research reveals multiple value streams that collectively deliver compelling business cases [81].
Table 4: Circular Economy Implementation ROI Analysis
| Value Stream | Financial Impact | Implementation Timeline | Key Performance Indicators |
|---|---|---|---|
| Material Cost Reduction | 15-35% raw material savings | Short-term (0-12 months) | Material circularity indicator, virgin material displacement |
| New Revenue Generation | 12-18% additional product revenue | Medium-term (12-36 months) | Circular product sales, service revenue growth |
| Risk Mitigation | 63% less supply chain disruption | Medium-term (12-36 months) | Supply chain resilience index, commodity price volatility exposure |
| Operational Efficiency | 25-30% maintenance cost reduction | Short-term (0-18 months) | Asset utilization rates, downtime reduction |
| Brand Value Enhancement | 27% higher brand valuation | Long-term (24+ months) | Brand sustainability perception, premium pricing capability |
Material cost reductions represent the most immediate financial benefit, with companies implementing circular procurement strategies achieving 15-35% raw material cost savings [81]. The Ellen MacArthur Foundation reports that consumer goods companies replacing virgin materials with recycled alternatives save an average of $2.8 million annually per $100 million in revenue [81]. Additionally, circular economy strategies significantly reduce supply chain vulnerability to commodity price volatility and geopolitical disruptions. Companies with diversified material sources including recycled content experienced 63% less supply chain disruption during 2023-2024 geopolitical events compared to those dependent solely on virgin materials [81].
The integration of circular economy principles in manufacturing represents both an urgent sustainability imperative and a significant economic opportunity. As the Nobel Declaration emphasizes, "We don't need to be facing these crises. We don't need to have a growing climate crisis. We don't need to have a biodiversity crisis. We don't need to have a forever chemicals crisis. We don't need to have any of these things because we have the solutions" [70]. The experimental protocols and implementation frameworks presented in this technical guide provide researchers and manufacturing professionals with practical methodologies for advancing this transition.
Future research should focus on several critical areas: (1) integrating life cycle assessment and techno-economic analysis in material design; (2) enhancing digitalization for smart waste monitoring and logistics; (3) strengthening policy tools and public engagement to promote behavioral shifts; and (4) developing standards and certification systems for waste-derived products [84]. Additionally, the maturation of emerging technologies—including artificial intelligence for material sorting, blockchain for supply chain transparency, and advanced recycling processes—will address current technical barriers to implementation [81] [84].
The historical context of the sustainable chemistry movement reminds us that fundamental transformation of industrial systems requires both technological innovation and structural shifts across science, policy, and education [70]. By embracing circular economy principles, manufacturing industries can simultaneously address environmental challenges while unlocking substantial economic value, ultimately creating a more sustainable, resource-efficient, and resilient industrial foundation for future generations.
The global chemical enterprise stands at a pivotal moment in its history. Major changes are needed to ensure that chemistry is sustainable and able to deliver on its potential of helping to solve some of the biggest challenges to society, according to leading scientists [86]. The traditional chemistry approaches developed over the past two centuries have caused much unintentional harm to people and the planet, even as they delivered major breakthroughs and great wealth [86]. This recognition has catalyzed the sustainable chemistry movement, which aims to transform chemical processes from reliance on substances that are "toxic, depleting, rare, persistent, and explosive/flammable to substances that are healthful, renewable, distributed, plentiful, unreactive, and degradable" [86]. The recent Stockholm Declaration on Chemistry for the Future, signed by prominent scientists including Yale's Paul Anastas (considered the father of green chemistry) and Nobel laureate Ben Feringa, represents a formalization of this movement and serves as a urgent call for action across scientific, educational, and policy domains [86] [70]. Within this transformative context, addressing the critical workforce and skills gaps has emerged as a fundamental prerequisite for success.
Historical Context of Sustainable Chemistry
The evolution of sustainable chemistry represents a paradigm shift from pollution control to prevention at the molecular level. This movement has gained substantial momentum through key historical developments:
From End-of-Pipe Solutions to Molecular Design
The sustainable chemistry movement emerged as a proactive alternative to traditional environmental remediation approaches. Instead of focusing on managing waste and pollution after it has been created, sustainable chemistry aims to design chemical products and processes that reduce or eliminate the generation of hazardous substances [70]. This fundamental shift requires new thinking about molecular design, manufacturing processes, and lifecycle considerations.
Key Policy and Industry Drivers
- International Declarations: The Stockholm Declaration on Chemistry for the Future represents a recent milestone, stating that "the risk to people, prosperity, and the planet from inaction and preservation of the status quo is far greater than any risks that may be involved with transitioning to a 'new chemistry for sustainability' model" [86] [70].
- Legislative Frameworks: The U.S. Sustainable Chemistry Research and Development Act of 2019, which received bipartisan support, mandated federal coordination of sustainable chemistry activities and led to the development of the Federal Sustainable Chemistry Strategic Plan released in December 2024 [87].
- European Initiatives: The EU Chemical Strategy for Sustainability (CSS) launched in October 2020 introduced the concept of "Safe and Sustainable by Design" (SSbD) as a voluntary assessment framework integrating safety, circularity, and functionality considerations throughout the chemical lifecycle [88].
The Contemporary Skills Gap Analysis
Quantitative Assessment of Workforce Shortages
Recent analyses reveal critical shortages in key specialties required for the transition to sustainable chemistry. The following table summarizes the most pressing skill deficiencies identified across the chemical sector:
Table 1: Critical Skills Shortages in the Sustainable Chemistry Workforce
| Skill Category | Specific Specializations in Short Supply | Impact on Circular Economy |
|---|---|---|
| Chemical Process Engineering | Process design, optimization, and scale-up for sustainable processes | Limits development of efficient, scalable green manufacturing |
| Research & Development | Novel catalyst design, bio-catalysis, alternative feedstock development | Slows innovation in safer chemicals and materials |
| Metallurgical Processes | Resource recovery, urban mining, material separation techniques | Hinders closed-loop material cycles and resource efficiency |
| Environmental Engineering | Lifecycle assessment, chemical footprint analysis, risk assessment | Impedes comprehensive sustainability evaluation and management |
| Cross-disciplinary Integration | Systems thinking, lifecycle analysis, interdisciplinary collaboration | Barriers to implementing Safe and Sustainable by Design (SSbD) frameworks |
Data from a recent cross-sector report indicates that the UK consumes 15.3 tonnes of materials per person each year—roughly double what is considered sustainable—with over 90% of these materials lost to the economy at end-of-life [89]. Transitioning from this linear model to a circular economy depends critically on addressing these skill shortages.
Root Causes of the Skills Gap
Several interconnected factors contribute to the persistent skills gap in sustainable chemistry:
- Educational Pipeline Challenges: Financial pressures on higher education institutions threaten the supply of new talent, while existing curricula often fail to integrate the necessary interdisciplinary approaches [89].
- Rapidly Evolving Field: The pace of innovation in sustainable chemistry—including digital tools, novel materials, and assessment frameworks—outstrips the ability of traditional educational programs to adapt [53] [88].
- Insufficient Interdisciplinary Training: Sustainable chemistry requires integration of toxicology, lifecycle assessment, engineering, and policy perspectives alongside core chemistry knowledge [88].
- Workforce Diversity Limitations: Lack of diversity in the chemical sciences restricts the talent pool and innovative capacity needed to address sustainability challenges [87] [89].
Essential Competencies and Training Frameworks
Core Technical Competencies
Building a workforce capable of implementing sustainable chemistry principles requires development of specific technical competencies:
Table 2: Essential Technical Competencies for Sustainable Chemistry Professionals
| Competency Domain | Specific Skills and Knowledge Areas | Application Context |
|---|---|---|
| Green Chemical Design | Alternative feedstock development, molecular design for degradation, bio-based material synthesis | Development of safer alternatives to persistent chemicals like PFAS |
| Catalysis & Biocatalysis | Homogeneous and heterogeneous catalysis, enzymatic synthesis, catalyst recovery and reuse | Efficient synthesis with reduced energy requirements and waste generation |
| Digital and AI Tools | Chemical informatics, predictive toxicology, lifecycle assessment software, process modeling | Accelerated screening and design of sustainable chemicals and processes |
| Circular Economy Principles | Chemical recycling technologies, waste valorization, design for reuse and recycling | Creating closed-loop systems that minimize resource extraction and waste |
| Analytical and Monitoring Techniques | Advanced mass spectrometry, bioanalytical methods, environmental fate tracking | Identification and assessment of transformation products and environmental impacts |
Implementation Methodologies
Hazard Screening and Assessment Workflow
Advanced hazard screening methodologies combine computational and experimental approaches to enable early-stage assessment of chemical safety. The following workflow illustrates the integrated approach:
Integrated Hazard Screening Workflow for Sustainable Chemistry
This workflow enables researchers to:
- Apply in silico tools using advanced machine learning and AI-based methods focusing on human endpoints such as mutagenesis, eye irritation, cardiovascular disease, and hormone disruption [88].
- Utilize conformal prediction theory providing uncertainty parameters and applicability domain measures per model and prediction [88].
- Implement experimental validation through in vitro and bioanalytical methods to confirm computational predictions.
- Integrate exposure assessment using advanced analytical workflows for time-efficient screening of broad chemical classes in environmental samples [88].
Sustainable Molecular Design Protocol
The development of safer chemicals requires systematic protocols that integrate sustainability considerations at the molecular design stage:
Sustainable Molecular Design Protocol
This protocol emphasizes:
- Forward-looking degradation modeling to predict environmental fate and transformation products during molecular design.
- Integrated hazard assessment combining computational toxicology and experimental validation.
- Early-stage lifecycle thinking incorporating techno-economic analysis and material flow considerations.
Research Reagent Solutions for Sustainable Chemistry
Table 3: Essential Research Reagents and Tools for Sustainable Chemistry Laboratories
| Reagent/Tool Category | Specific Examples | Function in Sustainable Chemistry |
|---|---|---|
| Bio-based Catalysts | Immobilized enzymes, engineered biocatalysts, microbial systems | Enable selective transformations under mild conditions with reduced energy requirements |
| Alternative Solvents | Ionic liquids, supercritical CO₂, bio-based solvents, water | Replace volatile organic compounds and hazardous solvents in chemical processes |
| Renewable Feedstocks | Algal oils, agricultural waste streams, captured CO₂ | Provide alternatives to fossil-based raw materials, enabling carbon circularity |
| Analytical Standards | PFAS alternatives, biodegradable polymer references, metabolite standards | Enable detection and quantification of sustainable chemical alternatives and their transformation products |
| Computational Tools | AI-based prediction platforms, lifecycle assessment software, molecular modeling suites | Accelerate design and evaluation of sustainable chemicals without resource-intensive laboratory work |
Educational Pathways and Program Innovations
Global Graduate Program Landscape
The educational landscape for sustainable chemistry training has expanded significantly, with diverse programs emerging worldwide:
Table 4: Representative Graduate Programs in Green and Sustainable Chemistry
| Institution | Program Type | Program Focus | Unique Features |
|---|---|---|---|
| Yale University | Professional Certificate | Green Chemistry for Climate and Sustainability | Online format; focuses on defossilization and hazard reduction; for professionals without extensive chemistry background [90] |
| Chulalongkorn University | PhD/MSc | Green Chemistry and Sustainability | Electives in Chemical Toxicology, Green Technology, Innovation Management [91] |
| University of York | MSc | Green Chemistry & Sustainable Industrial Technology | Systems thinking approach; includes business case development and communication strategies [91] |
| Monash University | Graduate Certificate | Green Chemistry & Sustainable Technologies | Focus on industrial transformation; includes consultancy project and safer chemical design [91] |
| Vienna University of Technology | MSc | Green Chemistry | Covers renewables, environmental analytical chemistry, and toxicology [91] |
| Stockholm University | MSc | Sustainable Chemistry | Focus on materials chemistry for environmental applications and recycling chemistry [91] |
| University of Massachusetts | PhD Track | Green Chemistry | Combines introduction to green chemistry with environmental toxicology [91] |
Curriculum Innovation Priorities
Bridging the skills gap requires fundamental redesign of chemistry education to incorporate:
- Interdisciplinary Coursework: Programs should allow students to develop awareness in areas that relate to sustainability, including lifecycle thinking, chemical toxicology, process engineering, economics, and communications [91].
- Problem-Solving and Design Focus: Professional opportunities require using multiple fundamental skillsets from the chemistry curriculum in tandem ("a systems approach") to solve specific problems or design new chemicals and products [91].
- Project-Based Learning: Capstone experiences and internships that involve independently identifying problems and devising appropriate solutions prepare graduates for real-world sustainability challenges [91].
- Digital Literacy Integration: Training in AI-driven chemical design, computational toxicology, and lifecycle assessment tools prepares students for modern sustainable chemistry practice [53] [88].
Implementation Strategies and Future Outlook
Multi-stakeholder Collaboration Frameworks
Addressing the workforce gap requires coordinated action across sectors:
- Industry-Academia Partnerships: Programs like the Mistra SafeChem research programme in Sweden demonstrate the value of collaboration between 6 research institutions and 14 companies representing industries from basic chemical and pharmaceutical producers to automobile producers and cosmetics suppliers [88].
- Professional Body Engagement: Organizations including the Royal Society of Chemistry, Institution of Chemical Engineers, and Institute of Materials, Minerals and Mining play critical roles in defining competency standards and promoting continuous professional development [89].
- Policy-Education Alignment: Government policies on chemical enterprise must be aligned with advancing healthful and safe chemistry, including tax incentives and subsidies that support workforce development [86] [87].
Emerging Technology Integration
Future skills development must anticipate technological trends transforming sustainable chemistry:
- AI and Machine Learning: These tools are being used to predict molecular behavior, bioaccumulation of chemicals in various organisms, and to accelerate simulations for more effective chemical design [92] [88].
- High-Throughput Experimentation: Automated science initiatives and high-throughput capabilities enable rapid evaluation of chemical properties and transformation pathways [92].
- Digital Twins: Virtual copies of physical assets allow operators to test process changes before implementation, enhancing safety and efficiency while reducing energy use and waste [53].
- Blockchain for Transparency: Digital tracking technologies enhance ESG reporting and regulatory compliance through supply chain transparency [53].
The transition to sustainable chemistry represents both an unprecedented challenge and opportunity for the global chemical enterprise. As articulated in the Stockholm Declaration, "the risk to people, prosperity, and the planet from inaction and preservation of the status quo is far greater than any risks that may be involved with transitioning to a 'new chemistry for sustainability' model" [86]. Addressing the critical workforce and skills gaps through educational innovation, interdisciplinary training, and multi-stakeholder collaboration is not merely an educational concern but a fundamental prerequisite for achieving a sustainable chemical industry. The success of this transformation depends on developing a new generation of chemists equipped with the technical competencies, systems thinking capabilities, and innovative mindset required to design molecular solutions that simultaneously advance product function, environmental protection, and economic viability. As the field continues to evolve, ongoing assessment and adaptation of training approaches will be essential to ensure the chemical workforce can meet the complex sustainability challenges of the 21st century.
The sustainable chemistry movement, born from a paradigm shift toward pollution prevention in the 1990s, has evolved to embrace lifecycle thinking (LCT) as its core philosophical framework [16] [18]. This approach moves beyond the traditional focus on production sites to include the environmental, social, and economic impacts of a product over its entire lifecycle, from raw material extraction to end-of-life disposal or recycling [93]. For researchers, scientists, and drug development professionals, this holistic view is critical for accurately assessing the true environmental footprint of pharmaceuticals and medical technologies.
Concurrently, the rise of Environmental, Social, and Governance (ESG) reporting has created a standardized mechanism for communicating sustainability performance to stakeholders, including investors, regulators, and the public [94]. Modern frameworks like the European Union's Corporate Sustainability Reporting Directive (CSRD) now explicitly require a lifecycle perspective for environmental disclosures [95]. Digital tools, particularly those enabling Life Cycle Assessment (LCA), provide the scientific and methodological foundation to bridge these domains, transforming qualitative sustainability goals into quantifiable, verifiable data [96] [95]. This technical guide explores the integration of these elements, providing a roadmap for employing digital LCA tools to meet rigorous ESG reporting standards within the context of pharmaceutical research and development.
Historical Context: From Green Chemistry Principles to Comprehensive Lifecycle Management
The conceptual foundation for sustainable chemistry was laid by the U.S. Pollution Prevention Act of 1990, which championed improved design to eliminate pollution at its source, moving away from "end-of-pipe" treatment and control strategies [16] [54]. The field was formally crystallized in 1998 with the publication of Paul Anastas and John Warner's 12 Principles of Green Chemistry, which provided a clear set of design guidelines for reducing or eliminating the use and generation of hazardous substances in chemical products and processes [16] [18].
Initially, regulatory efforts focused on single-issue environmental problems, such as the toxicity risks of pharmaceutical substances assessed in Environmental Risk Assessments (ERAs) [93]. However, this narrow scope proved insufficient for capturing the multidimensional nature of environmental impacts, which include greenhouse gas (GHG) emissions, resource depletion, water pollution, and biodiversity loss across a product's entire value chain [93]. This recognition spurred the adoption of lifecycle thinking, a more comprehensive approach that considers all stages of a product's lifecycle and a broader range of environmental impact categories [93] [97].
The legislative journey of the Sustainable Chemistry Research and Development Act in the United States, ultimately passed in 2021, highlights the growing political recognition of this integrated approach and the need for coordinated government leadership to overcome barriers to innovation in safer, more sustainable chemicals [54].
Core Methodologies: Life Cycle Assessment (LCA) and ESG Reporting Standards
The Life Cycle Assessment (LCA) Framework
Life Cycle Assessment (LCA) is a standardized, systematic methodology for evaluating the environmental impacts associated with all stages of a product's life, from cradle to grave [96]. The International Organization for Standardization (ISO) provides the governing standards for LCA (ISO 14040 and 14044), which structure the process into four distinct, interdependent phases [96] [95]:
- Goal and Scope Definition: This initial phase defines the purpose of the study, the product system to be assessed, the functional unit (a quantifiable measure of performance), and the system boundaries (e.g., cradle-to-gate vs. cradle-to-grave) [96] [95].
- Life Cycle Inventory (LCI) Analysis: This involves creating a detailed inventory of all material and energy inputs (e.g., raw materials, water, energy) and environmental outputs (e.g., emissions to air, water, soil) associated with the product system throughout its lifecycle [95].
- Life Cycle Impact Assessment (LCIA): In this phase, the inventory data is translated into potential environmental impacts using predefined impact categories, such as global warming potential, eutrophication, acidification, and water use [96] [95].
- Interpretation: The findings from the LCI and LCIA are summarized and discussed to provide conclusions, limitations, and recommendations in the context of the defined goal and scope [96].
Table 1: Common Life Cycle Models in LCA
| Model | Scope | Common Use Case |
|---|---|---|
| Cradle-to-Grave | Raw material extraction to final disposal | Comprehensive product footprinting [96] |
| Cradle-to-Gate | Raw material extraction to factory gate | Environmental Product Declarations (EPDs) for business-to-business communication [96] |
| Cradle-to-Cradle | Raw material extraction to recycling into a new product | Circular economy and closed-loop systems [96] |
| Gate-to-Gate | A single value-added process in the production chain | Assessing specific manufacturing processes within a larger chain [96] |
ESG Reporting Frameworks and Standards
ESG reporting provides a structured framework for companies to disclose performance on environmental, social, and governance criteria [94]. For the environmental pillar, which is the focus of this guide, several key mandatory and voluntary frameworks exist:
- Corporate Sustainability Reporting Directive (CSRD): A mandatory framework in the European Union requiring detailed disclosures according to the European Sustainability Reporting Standards (ESRS) [94] [95]. The ESRS includes specific standards on climate change (E1), pollution (E2), water and marine resources (E3), biodiversity (E4), and resource use and circular economy (E5) [95].
- International Sustainability Standards Board (ISSB): Develops global baseline standards for sustainability disclosures, such as IFRS S1 and S2, to facilitate consistent reporting across jurisdictions [94].
- Global Reporting Initiative (GRI): A widely adopted voluntary framework providing comprehensive standards for reporting on a broad range of sustainability topics [94].
- Task Force on Climate-Related Financial Disclosures (TCFD): Focuses specifically on climate-related financial risks and opportunities [94].
The EU Taxonomy is a classification system that complements the CSRD by defining which economic activities can be considered environmentally sustainable, guiding capital toward truly green investments [98] [95].
The Digital Integration: How LCA Tools Feed into ESG Reporting
Digital LCA software platforms are the technological linchpins that make robust, data-driven ESG reporting feasible. They enable the complex data collection, modeling, and calculation required to generate the quantitative environmental data demanded by frameworks like the ESRS.
The logical workflow below illustrates how digital LCA processes are integrated to support ESG compliance, translating product-level data into corporate sustainability reports.
The connection is further solidified by the Product Environmental Footprint (PEF), an LCA-based method promoted by the European Commission [95]. The PEF provides a standardized methodology for calculating a product's environmental footprint across 16 impact categories, creating a direct, science-based link between product-level LCA results and the corporate-level disclosures required by the ESRS [95].
Table 2: Linking LCA Impact Categories to ESRS Environmental Disclosure Topics
| LCA Impact Category (Example) | Relevant ESRS Disclosure Topic | Quantitative Data Provided via LCA |
|---|---|---|
| Global Warming Potential | ESRS E1 - Climate Change | GHG Emissions (Scopes 1, 2, 3) [95] |
| Water Use/Source Depletion | ESRS E3 - Water and Marine Resources | Water consumption (m³) [98] [95] |
| Eutrophication Potential | ESRS E2 - Pollution | Releases of pollutants to water [95] |
| Resource Depletion (Fossil, Mineral) | ESRS E5 - Resource Use and Circular Economy | Resource efficiency, material footprint [95] |
| Land Use | ESRS E4 - Biodiversity and Ecosystems | Impacts on biodiversity and ecosystems [95] |
A Pharmaceutical and Drug Development Case Study
The application of digital LCA for ESG reporting is highly relevant to the pharmaceutical sector, which faces increasing scrutiny of its environmental footprint, particularly concerning GHG emissions from anesthesia and inhalers, as well as water pollution from API manufacturing [93].
Consider a real-world example inspired by recent regulatory actions: evaluating the lifecycle impact of desflurane, a volatile anesthetic gas, and alternatives [93]. NHS England decided to stop the routine use of desflurane by early 2024, a policy supported by a NICE Evidence Summary that highlighted its high global warming potential [93]. A digital LCA tool can quantify this impact and provide the necessary data for an ESG report.
The following workflow diagram details the specific experimental and data management protocol for conducting such an assessment.
Table 3: Key Research Reagent Solutions for Sustainable Chemistry & LCA
| Tool / Reagent Category | Function in Sustainable Chemistry & LCA |
|---|---|
| Digital LCA Software Platforms | Software tools that automate lifecycle inventory modeling and impact calculation, enabling scenario analysis and data management for ESG reporting [96]. |
| Bio-based & Green Solvents | Solvents derived from renewable resources (e.g., soybean oil polyols) designed to replace fossil-based solvents, reducing toxicity and lifecycle carbon footprint [18] [95]. |
| Catalysts (e.g., Metathesis Catalysts) | Catalysts that enable more efficient synthetic routes with fewer steps, less energy, and reduced waste, a core principle of green chemistry [16]. |
| Green Chemistry Metrics Calculators | Digital tools that calculate metrics like Atom Economy, Process Mass Intensity, and E-factor to quantitatively evaluate the greenness of synthetic protocols [16]. |
| ESG Data Management Platforms | Systems that centralize the collection, aggregation, and management of quantitative and qualitative ESG data from multiple sources for streamlined reporting [94]. |
The integration of lifecycle thinking, powered by digital LCA tools, into ESG reporting represents a maturation of the sustainable chemistry movement from a conceptual framework to an actionable, data-driven enterprise. For drug development professionals and researchers, this integration is no longer optional but a core component of regulatory compliance, market access, and corporate responsibility [93] [94] [95].
The future will likely see a tightening of this integration, with LCA becoming the default methodological backbone for environmental disclosures under frameworks like the CSRD. Advancements in predictive toxicology, digital product passports, and AI-powered supply chain traceability will further enhance the precision and scope of digital LCAs [16] [18]. By adopting these tools and methodologies now, scientists and organizations in the pharmaceutical sector can not only meet their immediate reporting obligations but also drive meaningful innovation, minimize regrettable substitutions, and genuinely contribute to a more sustainable and healthier future.
Proof of Concept: Validating Impact Through Awards, Case Studies, and Emerging Trends
The Presidential Green Chemistry Challenge Awards stand as a premier recognition of innovations that advance the integration of sustainability into chemical practice. For the pharmaceutical industry, these awards highlight a critical evolution: the shift from viewing green chemistry as a regulatory burden to embracing it as a strategic imperative for economic viability, enhanced safety, and reduced environmental impact. The pharmaceutical industry faces immense sustainability challenges, with global active pharmaceutical ingredient (API) production generating an estimated 10 billion kilograms of waste annually at a disposal cost of approximately $20 billion [99]. Within this context, the award-winning technologies provide a blueprint for reconciling the demands of drug development with the principles of environmental stewardship. This analysis examines the documented successes of pharmaceutical winners, distilling the technical methodologies and strategic frameworks that have demonstrated commercial and ecological success. By framing these achievements within the broader historical context of the sustainable chemistry movement, this review aims to equip researchers and drug development professionals with the knowledge to further innovate and implement green chemistry principles across the pharmaceutical lifecycle.
Historical Context and Evolution of the Awards
The Presidential Green Chemistry Challenge Awards were established in 1996, a pivotal moment in the formalization of green chemistry as a scientific discipline [16] [9]. This initiative emerged from a paradigm shift in U.S. environmental policy, notably the Pollution Prevention Act of 1990, which championed pollution prevention at the source over end-of-pipe cleanup [16]. The program was initially administered by the U.S. Environmental Protection Agency (EPA) and now, reflecting its deep integration into the scientific community, is run by the American Chemical Society (ACS) [100].
The awards have consistently evolved to address emerging scientific and environmental priorities. For 2026, the program features refined categories, including a dedicated "Greener Synthetic Pathway in the Synthesis of Pharmaceuticals," signaling the continued importance of the pharmaceutical sector in advancing green chemistry [100]. This historical progression underscores a growing recognition that designing inherently safer and more efficient processes is not only beneficial for the environment but also drives innovation and economic competitiveness in the chemical industries.
Analysis of Award-Winning Pharmaceutical Technologies
The following analysis synthesizes the methodologies and achievements of recent award-winning technologies from pharmaceutical companies, highlighting the practical application of green chemistry principles.
Table 1: Recent Presidential Green Chemistry Challenge Award Winners in Pharmaceuticals
| Award Year | Company/Academic Institution | Award Category | Technology Summary | Key Green Chemistry Principles Demonstrated |
|---|---|---|---|---|
| 2024 | Merck & Co. Inc. | Greener Synthetic Pathways | Continuous manufacturing automated process for pembrolizumab (KEYTRUDA) [31]. | Waste Prevention; Design for Energy Efficiency; Safer Solvents; Catalysis |
| 2022 | Merck & Co. Inc. | Greener Synthetic Pathways | Greener synthesis of molnupiravir (LAGEVRIO), an antiviral COVID-19 treatment [31]. | Atom Economy; Less Hazardous Synthesis; Reduce Derivatives |
| 2022 | Amgen | Greener Reaction Conditions | Improved manufacturing process for sotorasib (LUMAKRAS), a treatment for non-small cell lung cancer [31]. | Safer Solvents and Auxiliaries; Energy Efficiency; Catalysis |
| 2021 | Bristol Myers Squibb | Greener Reaction Conditions | Development and implementation of five sustainable reagents [31]. | Design of Safer Chemicals; Inherently Safer Chemistry |
| 2020 | Merck & Co. | Greener Reaction Conditions | Multifunctional catalyst for the stereoselective assembly of ProTide prodrugs [31]. | Catalysis; Atom Economy; Reduce Derivatives |
| 2019 | Merck & Co. | Greener Synthetic Pathways | Sustainable commercial manufacturing process for letermovir [31]. | Waste Prevention; Less Hazardous Synthesis; Safer Solvents |
| 2017 | Amgen Inc. / Bachem | Greener Reaction Conditions | Green process for the commercial manufacture of Etelcalcetide using improved solid-phase peptide synthesis technology [31]. | Atom Economy; Safer Solvents and Auxiliaries; Energy Efficiency |
Detailed Methodologies and Experimental Protocols
Continuous Flow Synthesis and Manufacturing
- Protocol Overview: Transitioning from traditional batch processing to a continuous flow system for API synthesis [101].
- Detailed Workflow:
- Reagent Preparation: Pharmaceutical starting materials and catalysts are dissolved in appropriate green solvents (e.g., 2-MethylTetrahydrofuran, Cyrene) and loaded into separate feed streams.
- Continuous Reaction: The feed streams are pumped at precisely controlled rates into a continuous flow reactor. The reactor consists of a narrow-diameter tube or a series of micro-structured modules, allowing for highly efficient heat and mass transfer.
- In-line Monitoring and Analysis: The reaction mixture is monitored in real-time using PAT tools such as inline IR or UV spectroscopy. This enables immediate feedback and control over critical process parameters (CPPs) to ensure quality.
- Continuous Work-up and Isolation: The output stream may be directly fed into in-line separation units (e.g., a liquid-liquid separator or a continuous crystallizer) for purification and isolation of the intermediate or final API.
- Key Technical Parameters: The system operates with highly controlled residence times (seconds to minutes), superior temperature control (± 2°C), and elevated pressures to maintain solvents in a liquid state at higher temperatures [101].
Biocatalysis and Enzyme Engineering
- Protocol Overview: Employing engineered enzymes as highly selective biocatalysts to perform specific chemical transformations under mild conditions [101].
- Detailed Workflow:
- Enzyme Selection and Engineering: A library of enzymes (e.g., ketoreductases, transaminases, nitrilases) is screened for the desired activity. Enzymes with suboptimal properties are engineered via directed evolution to improve activity, stability, or selectivity.
- Reaction Setup: The biocatalytic reaction is performed in an aqueous buffer or a biphasic system (buffer/green solvent) at a pH and temperature optimal for the enzyme (typically 20-40°C, pH 6-8).
- Cofactor Regeneration: For reactions requiring cofactors (e.g., NADPH), an efficient cofactor regeneration system is integrated to avoid stoichiometric use and reduce cost.
- Product Recovery: The product is isolated via extraction, crystallization, or, in a flow system, using an in-line membrane separator.
- Key Technical Parameters: The protocol focuses on high atom economy, minimal protection/deprotection steps, and reactions conducted at ambient temperature and pressure [101].
Technical Workflow and Strategic Implementation Framework
The development and implementation of a award-winning green pharmaceutical process requires a systematic approach that integrates molecular design, process engineering, and analytical control. The following workflow and toolkit outline the critical components for success.
Strategic Implementation Workflow
The pathway from initial concept to a commercially viable green manufacturing process involves several interconnected stages, as visualized below.
Diagram 1: Green Chemistry Process Development Workflow.
This workflow underpins the development of many award-winning processes. For instance, Merck's continuous process for pembrolizumab and Pfizer's improved synthesis of sertraline both exemplify this systematic approach, resulting in doubled yield, significant reduction of hazardous materials, and lower energy consumption [31] [102].
The Scientist's Toolkit: Essential Research Reagents and Materials
The successful implementation of green chemistry in pharmaceutical research and development relies on a suite of specialized reagents, catalysts, and solvents designed to reduce environmental impact while maintaining efficiency.
Table 2: Key Research Reagent Solutions for Green Pharmaceutical Chemistry
| Reagent/Material | Function in Green Chemistry | Example Application |
|---|---|---|
| Immobilized Enzymes | Biocatalysts for selective reactions under mild conditions; reusable, reducing waste [101]. | Asymmetric ketone reduction for chiral alcohol synthesis. |
| Earth-Abundant Metal Catalysts | Replace scarce and toxic heavy metal catalysts (e.g., palladium) in cross-couplings and other transformations [31]. | Iron-catalyzed C-C cross-coupling reactions. |
| Bio-Based Solvents | Replace petroleum-derived, hazardous solvents; derived from renewable feedstocks (e.g., 2-MeTHF, Cyrene, limonene) [101] [99]. | Solvent for extraction and reaction media, improving process safety. |
| Solid-Supported Reagents | Facilitate purification (filtration), can be used in excess and recycled, minimizing aqueous waste streams [31]. | Oxidation reactions where the spent reagent is easily removed by filtration. |
| Continuous Flow Reactor Systems | Enable process intensification, improve heat/mass transfer, enhance safety, and reduce solvent use [101] [102]. | Synthesis of highly energetic or exothermic intermediates. |
The Presidential Green Chemistry Challenge Award winners in the pharmaceutical sector provide compelling, real-world evidence that sustainable molecular design and process engineering are not only feasible but also commercially advantageous. The documented successes—from Merck's continuous manufacturing to the widespread adoption of biocatalysis—demonstrate significant progress in reducing the environmental footprint of drug production. These innovations, which prevent waste at the source and employ safer, energy-efficient processes, represent a tangible realization of the principles that have guided the green chemistry movement since its formal inception in the 1990s.
For researchers, scientists, and drug development professionals, these case studies serve as both inspiration and a practical guide. The continued integration of artificial intelligence for reaction optimization and the systematic application of circular economy principles will further accelerate this transition [101] [99]. Embracing this mindset is no longer a niche pursuit but a fundamental component of modern, responsible, and innovative pharmaceutical development. The future of drug manufacturing lies in continuing to build upon this legacy of success, ensuring that the pursuit of human health is intrinsically linked to the health of our planet.
The Nobel Prize in Chemistry, in both 2001 and 2005, served as a definitive ratification of green chemistry's core principles, elevating it from a specialized niche to a mainstream scientific paradigm. The 2001 prize recognized catalytic asymmetric synthesis, a methodology that inherently promotes atom economy and reduces waste. The 2005 prize honored olefin metathesis, a transformative reaction that emphasizes energy efficiency and the design of safer syntheses. This whitepaper details the technical underpinnings of these award-winning discoveries, provides protocols for their implementation, and analyzes their profound impact within the historical context of the sustainable chemistry movement. Directed at researchers and drug development professionals, this review underscores how Nobel-endorsed innovations are instrumental in designing more efficient and environmentally benign chemical processes.
The modern green chemistry movement, formalized by Paul Anastas and John Warner in their 1998 twelve principles, represents a paradigm shift from pollution cleanup to pollution prevention [18]. Its core tenets include waste minimization, safer solvent use, catalytic efficiency, and reduced energy consumption. For decades, however, the field sought a definitive signal of its central importance to chemical science.
This recognition arrived authoritatively through the Nobel Prizes in Chemistry in 2001 and 2005. The Royal Swedish Academy of Sciences explicitly highlighted the green credentials of these discoveries, noting that metathesis represents "a great step forward for 'green chemistry,' reducing potentially hazardous waste through smarter production" [103]. The recognition of these fields was not merely for their synthetic elegance but for their alignment with a preventative environmental philosophy that had been coalescing since the 1960s, spurred by events like the publication of Silent Spring and the establishment of the US Environmental Protection Agency [9] [18].
The following sections provide a technical dissection of these Nobel-prize winning reactions, offering researchers a guide to their application, their quantifiable green credentials, and their enduring legacy in legitimizing sustainable research.
The 2001 Nobel Prize in Chemistry: Catalytic Asymmetric Synthesis
Award-Winning Work and Green Chemistry Principles
The Nobel Prize in Chemistry 2001 was awarded with one half jointly to William S. Knowles and Ryoji Noyori and the other half to K. Barry Sharpless. Knowles and Noyori were recognized for their work on chirally catalysed hydrogenation reactions, while Sharpless was honored for his work on chirally catalysed oxidation reactions [104]. Their collective work on catalytic asymmetric synthesis directly advanced several green chemistry principles, most notably Atom Economy (Principle #2) and the Use of Catalysis (Principle #9) [105].
The fundamental challenge addressed by the laureates was the efficient synthesis of single-enantiomer molecules. Many pharmaceuticals and biologically active compounds are chiral, meaning they exist as two non-superimposable mirror images, much like a pair of hands [106]. While nature typically produces only one of these forms, traditional chemical synthesis creates a 50:50 mixture, known as a racemate. Knowles, Noyori, and Sharpless developed catalytic methods to produce a predominance of the desired enantiomer, thereby avoiding the wasteful synthesis and subsequent separation of inactive or harmful mirror-image molecules [106].
Detailed Experimental Protocols
Protocol 1: Knowles' Asymmetric Hydrogenation for L-DOPA Synthesis
Objective: To synthesize L-DOPA (L-3,4-dihydroxyphenylalanine), a drug used to treat Parkinson's disease, via asymmetric hydrogenation [106].
Materials:
- Substrate: (Z)-acylamido cinnamic acid derivative.
- Chiral Catalyst: Rhodium(I) complex with a chiral diphosphine ligand (e.g., DiPAMP, developed by Monsanto).
- Hydrogen gas (H₂).
- Solvent: Methanol or ethanol.
Procedure:
- Reaction Setup: Charge a pressure-resistant reaction vessel with the substrate and the chiral rhodium catalyst (typically 0.1-1.0 mol%).
- Dissolution: Add degassed solvent to the vessel under an inert atmosphere (e.g., nitrogen or argon) to prevent catalyst decomposition.
- Hydrogenation: Pressurize the vessel with H₂ gas to 5-10 atm. Stir the reaction mixture at room temperature for 6-24 hours.
- Monitoring: Monitor reaction progress by TLC or HPLC until the starting material is consumed.
- Work-up: Upon completion, release the hydrogen pressure and concentrate the reaction mixture under reduced pressure.
- Isolation: Purify the product via crystallization. The chiral product precipitates, while the catalyst remains in the mother liquor.
- Hydrolysis: Treat the purified intermediate with aqueous hydrochloric acid to remove the acyl protecting group, yielding L-DOPA.
Key Green Chemistry Metrics: This process, the first industrial catalytic asymmetric synthesis, achieved 97.5% enantiomeric excess (e.e.) in the hydrogenation step [106]. It exemplifies atom economy by incorporating the entire substrate and hydrogen molecules into the product, minimizing waste.
Protocol 2: Sharpless Asymmetric Epoxidation
Objective: To convert a primary allylic alcohol into an epoxy alcohol with high enantioselectivity [106].
Materials:
- Substrate: Allylic alcohol.
- Catalysts: Titanium(IV) isopropoxide (Ti(OiPr)₄) and a chiral tartrate ester (e.g., Diethyl D- or L-tartrate (DET or LET)).
- Stoichiometric Oxidant: tert-Butyl hydroperoxide (TBHP).
- Solvent: Dichloromethane (Note: modern green adaptations seek to replace this solvent).
- Molecular sieves (4Å).
Procedure:
- Activation of Molecular Sieves: Flame-dry molecular sieves under vacuum to ensure strict anhydrous conditions.
- Catalyst Formation: In the reaction vessel, combine the chiral tartrate ester (1.0-1.2 equiv) and Ti(OiPr)₄ (1.0-1.2 equiv) in dry CH₂Cl₂ at -20°C. Stir for 30-60 minutes to form the chiral titanium-tartrate complex.
- Substrate Addition: Add the allylic alcohol (1.0 equiv) to the catalyst mixture.
- Oxidation: Add tert-butyl hydroperoxide (TBHP, 1.0-1.5 equiv) dropwise to the stirring solution at -20°C.
- Reaction Monitoring: Maintain the temperature at -20°C and monitor by TLC until completion (typically 2-12 hours).
- Quenching: Carefully quench the reaction by adding a saturated aqueous solution of sodium sulfate (which also decomposes the titanium complex).
- Work-up and Purification: Filter the mixture through Celite, separate the organic layer, dry over anhydrous MgSO₄, and concentrate. Purify the epoxy alcohol product by flash chromatography.
Key Green Chemistry Metrics: This reaction is highly atom-economical and catalytic in chirality. The tartrate ligand is used in sub-stoichiometric quantities relative to the product, and the protocol allows for the predictable synthesis of complex chiral building blocks from simple starting materials.
Table 1: Quantitative Green Chemistry Metrics of 2001 Nobel Prize Reactions
| Reaction / Process | Catalyst System | Enantiomeric Excess (e.e.) | Key Green Chemistry Principle Demonstrated | Industrial Impact |
|---|---|---|---|---|
| Knowles' L-DOPA Synthesis | Rhodium-DiPAMP | 97.5% [106] | Atom Economy, Catalysis | First industrial asymmetric catalytic synthesis [106] |
| Noyori's BINAP Hydrogenation | Ruthenium-BINAP | >99% in many cases [106] | Atom Economy, Safer Solvents & Auxiliaries | Production of antibiotics, perfumes, and other fine chemicals |
| Sharpless Epoxidation | Titanium-Tartrate | Often >90% [106] | Catalysis, Reduce Derivatives | Key step in synthesis of complex pharmaceuticals like Tamiflu |
The 2005 Nobel Prize in Chemistry: The Metathesis Method in Organic Synthesis
Award-Winning Work and Green Chemistry Principles
The Nobel Prize in Chemistry 2005 was awarded jointly to Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock "for the development of the metathesis method in organic synthesis" [103] [107]. Olefin metathesis is a reaction in which double bonds between carbon atoms are broken and reformed, causing atom groups to change places in a process likened to a "change-your-partners dance" [103]. This method had a transformative impact on green chemistry by enabling more direct and efficient synthetic pathways.
The laureates' work directly advanced multiple green principles. Metathesis reactions are typically catalytic, with a single catalyst molecule generating millions of product molecules [103]. They often reduce the number of synthetic steps required to build complex molecules, leading to less resource consumption and waste generation [103] [108]. Furthermore, the development of stable, efficient catalysts by Schrock and Grubbs made these reactions simpler to use under milder, less energy-intensive conditions [103].
Detailed Experimental Protocols
Protocol 3: Ring-Closing Metathesis (RCM) using a Grubbs Catalyst
Objective: To form a cyclic olefin from a diene, a common step in the synthesis of natural products and pharmaceuticals.
Materials:
- Substrate: A diene (e.g., Diethyl diallyl malonate).
- Catalyst: Grubbs' 2nd generation catalyst.
- Solvent: Dichloromethane (or a greener alternative like toluene or EtOAc). The catalyst's stability in air allows for greater solvent choice [103].
- Inert Atmosphere: Nitrogen or argon.
Procedure:
- Reaction Setup: In a round-bottom flask equipped with a stir bar, dissolve the diene substrate (1.0 equiv) in dry, degassed solvent (0.1-0.5 M concentration).
- Catalyst Addition: Add the Grubbs catalyst (0.5-5.0 mol%) to the stirring solution at room temperature.
- Reflux: Heat the reaction mixture to reflux and monitor by TLC or GC-MS. The reaction is often complete within several hours. The evolution of ethylene gas provides a driving force.
- Completion and Quenching: Once the starting material is consumed, cool the reaction mixture to room temperature.
- Purification: Concentrate the mixture under reduced pressure and purify the crude product by flash chromatography on silica gel to yield the cyclic olefin.
Key Green Chemistry Metrics: This protocol demonstrates process intensification by constructing complex ring systems in a single step that might previously have required multiple derivatizations and purifications. The use of a highly active catalyst reduces the required loading, minimizing heavy metal waste.
Protocol 4: Cross Metathesis (CM) for Synthesis of Unsaturated Fatty Esters
Objective: To synthesize long-chain unsaturated esters from renewable seed oil derivatives.
Materials:
- Substrates: Olefin partners (e.g., methyl oleate and 1-decene).
- Catalyst: Hoveyda-Grubbs 2nd generation catalyst.
- Solvent: Toluene.
Procedure:
- Reaction Setup: Combine the two olefin substrates (often used in unequal molar ratios to drive selectivity) in a Schlenk flask.
- Catalyst Addition: Add the catalyst (0.1-1.0 mol%) to the mixture.
- Reaction: Stir the reaction mixture at 40-80°C under an inert atmosphere for 2-12 hours.
- Monitoring: Monitor by GC or TLC for consumption of the limiting reagent.
- Work-up: Remove the solvent under vacuum.
- Purification: Distill the residue under reduced pressure to separate the desired cross-metathesis product from unreacted starting materials and homodimer byproducts.
Key Green Chemistry Metrics: This process exemplifies the use of renewable feedstocks (seed oils) to create valuable chemicals, reducing reliance on petrochemical sources [108]. It is a key example of metathesis being used in the chemical industry for cleaner production.
Table 2: Evolution and Impact of Metathesis Catalysts (2005 Nobel Prize)
| Catalyst / Contributor | Key Characteristics | Stability & Handling | Green Chemistry Impact |
|---|---|---|---|
| Chauvin's Mechanism | Proposed metal-carbene mechanism (1971) [103] | N/A (Theoretical framework) | Provided the "recipe" for future catalyst design |
| Schrock Catalyst | First efficient well-defined catalyst (Mo-based, 1990) [103] | Air- and moisture-sensitive | Proved high activity was achievable; enabled new chemistry |
| Grubbs 1st Gen. | Ruthenium-based (1992) [103] | Stable in air | Broader functional group tolerance, easier for non-specialists to use |
| Grubbs 2nd Gen. | N-Heterocyclic carbene ligand [103] | Highly active, stable in air | More efficient (lower loadings, milder conditions), widely used in industry |
The Scientist's Toolkit: Essential Reagents and Materials
Table 3: Research Reagent Solutions for Nobel Prize-Winning Green Chemistry
| Reagent / Material | Function in Reaction | Green Chemistry Rationale | Example from Nobel Work |
|---|---|---|---|
| Chiral Diphosphine Ligands (e.g., DiPAMP, BINAP) | Binds to transition metal to create a chiral environment for asymmetric hydrogenation. | Enables high enantioselectivity, reducing waste of the incorrect enantiomer [106]. | Knowles (L-DOPA), Noyori (general hydrogenations) |
| Chiral Tartrate Esters (e.g., DET, LET) | Ligands for titanium to form the catalyst for asymmetric epoxidation. | Catalytic in chirality; inexpensive and derived from natural sources [106]. | Sharpless Epoxidation |
| Schrock Catalyst | Molybdenum-based alkylidene complex for olefin metathesis. | High-activity catalyst for C-C bond formation, enabling simpler synthetic routes [103]. | Ring-Closing Metathesis of complex molecules |
| Grubbs Catalysts (1st & 2nd Gen.) | Ruthenium-based alkylidene complexes for olefin metathesis. | Excellent functional group tolerance and stability in air; reduces need for stringent conditions [103] [108]. | Industrial-scale metathesis in pharmaceuticals and materials |
| Hydrogen Gas (H₂) | Stoichiometric reductant in hydrogenation reactions. | Ideal atom economy; produces no stoichiometric byproducts other than the desired product [106]. | Knowles and Noyori hydrogenations |
Reaction Mechanism and Workflow Visualizations
Asymmetric Hydrogenation Mechanism
Olefin Metathesis Catalytic Cycle
General Workflow for Catalytic Reactions
The Nobel Prizes of 2001 and 2005 were not merely awards for discrete chemical discoveries; they were a profound legitimization of the green chemistry ethos. By honoring asymmetric catalysis and metathesis, the Nobel Committee underscored that the most elegant and fundamental science is also that which is inherently more efficient, less wasteful, and environmentally responsible. These methodologies are now embedded in the toolkit of researchers and drug development professionals worldwide, enabling the synthesis of complex molecules with unprecedented precision and reduced environmental impact.
The legacy of these awards continues to shape the field. They provided a powerful impetus for academic and industrial research into greener catalytic processes and demonstrated that the principles of green chemistry are compatible with, and even essential for, cutting-edge scientific innovation and economic viability. As the chemical industry continues its transition toward sustainability, the catalytic strategies championed by these Nobel Laureates will remain foundational pillars of green molecular design.
The pharmaceutical industry stands at a critical juncture, balancing the imperative to develop effective therapeutics with the urgent need to minimize its environmental footprint. This analysis examines the evolution from traditional synthesis methods to green chemistry approaches for pharmaceutical intermediates, contextualized within the broader historical sustainable chemistry movement. The paradigm shift toward green chemistry represents a fundamental redesign of chemical synthesis, moving beyond pollution control to pollution prevention at the molecular level [16] [18]. The manufacturing, use, and disposal of pharmaceuticals have significant environmental ramifications, as residues and debris may infiltrate ecosystems, potentially causing harm and contributing to issues like antibiotic resistance [109]. The concept of green chemistry, formally defined by Paul Anastas and John Warner in the 1990s as "the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances," provides a framework for addressing these challenges while maintaining economic viability [109] [16] [18].
Historical Context of Sustainable Chemistry
The environmental movement that paved the way for green chemistry gained significant traction in the post-industrial era. Growing awareness of industrial pollution's consequences led to pivotal events such as the 1972 Stockholm Conference, which alerted the world to environmental damage from ecosystem depletion [18]. The 1987 "Brundtland Report" formally defined sustainable development as development meeting present needs without compromising future generations, emphasizing the dangers of ozone depletion and global warming [18].
The U.S. Pollution Prevention Act of 1990 established a national policy favoring pollution prevention over end-of-pipe treatment, creating the foundational policy context for green chemistry [16]. The field was formally articulated with the 1998 publication of the 12 Principles of Green Chemistry by Anastas and Warner, providing a comprehensive set of design guidelines [109] [16] [18]. The establishment of the Presidential Green Chemistry Challenge Awards in 1996 and the launch of the journal Green Chemistry in 1999 further institutionalized the field [16] [18]. This historical trajectory demonstrates how green chemistry emerged as a strategic response to systemic environmental challenges, evolving from conceptual foundations to practical implementation across the pharmaceutical industry and other chemical sectors.
The Twelve Principles of Green Chemistry
The 12 principles of green chemistry provide a systematic framework for designing chemical products and processes that reduce environmental impact and health hazards [109] [18]. These principles have remained highly relevant since their formulation and cover all aspects of a chemical process's life cycle:
- Prevention: It is better to prevent waste than to treat or clean up waste after it is formed.
- Atom Economy: Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
- Less Hazardous Chemical Syntheses: Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
- Designing Safer Chemicals: Chemical products should be designed to preserve efficacy of function while reducing toxicity.
- Safer Solvents and Auxiliaries: The use of auxiliary substances should be made unnecessary wherever possible and innocuous when used.
- Design for Energy Efficiency: Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized.
- Use of Renewable Feedstocks: A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
- Reduce Derivatives: Unnecessary derivatization should be minimized or avoided because such steps require additional reagents and can generate waste.
- Catalysis: Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
- Design for Degradation: Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment.
- Real-time Analysis for Pollution Prevention: Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
- Inherently Safer Chemistry for Accident Prevention: Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires.
Quantitative Comparison Framework
Environmental Factor (E-Factor) Analysis
A critical metric for evaluating the environmental impact of pharmaceutical synthesis is the E-Factor, introduced by Roger Sheldon, which quantifies the waste generated per kilogram of product [109]. The pharmaceutical industry has some of the highest E-Factors among chemical sectors, highlighting the urgent need for greener approaches.
Table 1: E-Factor Comparison Across Chemical Industries
| Industry Sector | E-Factor (kg waste/kg product) | Volume Production (annual) |
|---|---|---|
| Pharmaceuticals | 25 - 100+ | Low to medium |
| Fine chemicals | 5 - 50 | Medium |
| Bulk chemicals | <1 - 5 | High |
| Petrochemicals | ~0.1 | Very high |
Solvent Usage and Waste Generation
Solvents constitute between 80-90% of the total mass used in pharmaceutical manufacturing processes, making them a primary contributor to waste generation and environmental impact [109]. The transition to greener solvents represents a significant opportunity for improving the sustainability of pharmaceutical synthesis.
Table 2: Solvent Usage in Traditional vs. Green Synthesis
| Parameter | Traditional Synthesis | Green Synthesis | Environmental Benefit |
|---|---|---|---|
| Solvent Mass Percentage | 80-90% of total mass | Significantly reduced | Reduced waste generation |
| Solvent Types | Halogenated, volatile organic compounds | Water, supercritical CO₂, bio-based solvents | Reduced toxicity and ozone depletion |
| Solvent Recovery | Limited recovery, single-use | Closed-loop recycling systems | Resource conservation |
| Environmental Impact | High persistence, bioaccumulation | Readily biodegradable | Reduced ecosystem impact |
Case Study: Synthesis of a Common Pharmaceutical Intermediate
Traditional Synthesis Approach
The conventional synthesis of active pharmaceutical ingredients (APIs) and their intermediates has typically relied on multi-step processes with limited regard for environmental considerations. These approaches often employ stoichiometric reagents rather than catalytic systems, hazardous solvents with high environmental persistence, and energy-intensive reaction conditions with extended reaction times [109]. Traditional methods frequently generate substantial waste through protection-deprotection sequences, purification steps, and low atom economy transformations. The environmental impact of these processes extends beyond the immediate waste stream to include energy consumption for heating, cooling, and purification, as well as the carbon footprint associated with sourcing and disposing of hazardous materials [109].
Green Synthesis Alternatives
Microwave-Assisted Synthesis
Microwave-assisted technology represents a promising economical and energy-efficient method that is gaining popularity in pharmaceutical settings [109]. This approach offers several distinct advantages:
- Reduced Reaction Time: Various organic reactions can be completed in minutes rather than hours or days through microwave irradiation [109].
- Mechanism: Microwave heating converts electromagnetic energy into heat energy through ionic conduction and dipole polarization mechanisms [109].
- Energy Efficiency: Rapid volumetric heating enables more efficient energy transfer compared to conventional heating methods [109].
- Improved Selectivity: Specific absorption characteristics can lead to enhanced reaction selectivity and reduced byproduct formation [109].
In practice, microwave-assisted synthesis of five-membered nitrogen heterocycles (pyrroles, pyrrolidines, fused pyrazoles, etc.) has demonstrated cleaner results with shorter reaction times, higher final compound purity, and improved yields compared to conventional techniques [109]. The selection of reaction medium is crucial, with polar organic solvents such as DMF, DMA, DMSO, NMP, methanol, ethanol, and acetic acid being preferred due to their efficient absorption of microwave energy [109].
Catalytic Approaches and Atom Economy
Green chemistry emphasizes the use of catalytic systems over stoichiometric reagents to improve atom economy and reduce waste [109]. The principle of atom economy, one of the twelve core principles of green chemistry, advocates for synthetic methods that maximize the incorporation of starting materials into the final product [109]. Catalytic approaches enable transformations with significantly reduced environmental factors by:
- Minimizing or eliminating byproducts
- Reducing the need for purification steps
- Enabling milder reaction conditions
- Decreasing energy consumption
The integration of catalytic systems with microwave assistance represents a particularly powerful green synthesis strategy that combines the benefits of both approaches.
Experimental Protocols and Methodologies
Decision Framework for Synthesis Route Selection
The following workflow provides a systematic approach for researchers to select appropriate synthesis methodologies based on green chemistry principles:
Detailed Microwave-Assisted Synthesis Protocol
Objective: Synthesis of five-membered nitrogen heterocycles using microwave-assisted green chemistry approach [109].
Materials and Equipment:
- Microwave reactor system with temperature and pressure monitoring
- Polar solvents (ethanol, water, or other green solvents)
- Catalyst system (if applicable)
- Nitrogen heterocycle precursors
- Purification materials (minimal)
Procedure:
- Reaction Mixture Preparation: Combine precursors in appropriate green solvent at optimized concentration in microwave-compatible vessel.
- Catalyst Addition: Add catalytic amount of catalyst if required for transformation.
- Reactor Setup: Seal vessel and place in microwave reactor, ensuring proper temperature and pressure monitoring.
- Irradiation Parameters: Set microwave power and temperature parameters based on optimization studies (typically 100-150°C).
- Reaction Monitoring: Monitor reaction progress in real-time using appropriate analytical methods.
- Reaction Completion: Terminate irradiation once completion is achieved (typically minutes instead of hours/days).
- Product Isolation: Separate product using minimal workup procedures, favoring direct crystallization or extraction with green solvents.
- Purification: Employ minimal purification steps, potentially leveraging the high selectivity of microwave reactions to reduce need for extensive chromatography.
- Solvent Recovery: Implement solvent recovery systems for recycling in subsequent batches.
Key Advantages:
- Rapid Volumetric Heating: Enables faster reaction kinetics [109]
- Reduced Reaction Time: Minutes instead of hours or days [109]
- Higher Purity: Reduced byproduct formation [109]
- Improved Yield: Enhanced reaction efficiency [109]
- Energy Efficiency: Targeted energy delivery reduces overall consumption [109]
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents and Materials for Green Pharmaceutical Synthesis
| Reagent/Material | Function | Green Alternatives | Application Notes |
|---|---|---|---|
| Solvents | Reaction medium, extraction | Water, ethanol, ethyl acetate, supercritical CO₂ | Replace halogenated and volatile organic solvents; bio-based options preferred [109] |
| Catalysts | Enhance reaction efficiency, reduce waste | Heterogeneous catalysts, biocatalysts, organocatalysts | Prefer reusable, non-toxic catalytic systems over stoichiometric reagents [109] |
| Energy Sources | Drive chemical reactions | Microwave irradiation, ultrasound, mechanochemistry | Alternative energy sources reduce reaction times and improve efficiency [109] |
| Reagents | Transform functional groups | Atom-economical reagents, renewable feedstocks | Select reagents with minimal toxicity and maximum incorporation into product [109] |
| Analytical Tools | Monitor reaction progress | Real-time in-process monitoring, green analytical chemistry | Minimize sample preparation and solvent use in analysis [18] |
The transition from traditional to green synthesis routes for pharmaceutical intermediates represents both an environmental imperative and an economic opportunity. The historical context of the sustainable chemistry movement demonstrates a systematic evolution from pollution control to pollution prevention, with green chemistry principles providing a robust framework for designing intrinsically safer and more efficient synthetic processes. Quantitative metrics such as the E-Factor reveal the significant environmental burden of traditional pharmaceutical synthesis, while emerging technologies like microwave-assisted synthesis offer practical pathways to substantially reduce this impact. The multidimensional benefits of green chemistry approaches—including reduced waste generation, lower energy consumption, decreased solvent usage, and improved safety profiles—position them as essential components of sustainable drug development. As the pharmaceutical industry continues to align with the principles of green chemistry and the broader goals of the European Green Deal, the integration of these approaches will be critical for developing therapeutics that meet patient needs while minimizing environmental consequences across the entire product life cycle.
The chemical industry's traditional "take-make-waste" model has created significant socio-environmental challenges, emphasizing the urgent need for a shift toward sustainability [61]. In response, two complementary yet distinct frameworks have emerged: green chemistry and circular chemistry. While green chemistry, formalized in the 1990s, focuses primarily on reducing waste and pollution at the molecular level, circular chemistry represents a more recent paradigm that emphasizes resource efficiency, recycling, and systemic transformation of the entire chemical sector [110] [16] [111]. The concept of green chemistry originated as a response to the Pollution Prevention Act of 1990 in the United States, which declared that national policy should eliminate pollution by improved design instead of relying on treatment and disposal [16]. The field was fundamentally established with the publication of the 12 Principles of Green Chemistry by Paul Anastas and John Warner in 1998, providing a clear set of guidelines for designing chemical products and processes that reduce or eliminate the use and generation of hazardous substances [16] [111].
In contrast, circular chemistry has emerged more recently as an operationalization of circular economy principles within the chemical sector, aiming to transform it into a closed-loop, resource-efficient, and waste-free system [110]. This framework addresses the entire life cycle of chemical products and seeks to decouple analytical performance from resource consumption rates [110]. The evolution from green to circular chemistry represents a necessary paradigm shift for the sector to support global actions toward sustainable development, particularly in the context of the triple planetary crisis: climate change, biodiversity loss, and pollution [110]. This article provides a comprehensive technical comparison of these two frameworks, examining their philosophical foundations, practical applications, and implementation methodologies to guide researchers, scientists, and drug development professionals in navigating the transition toward sustainable chemistry practices.
Historical Context and Philosophical Foundations
The Development of Green Chemistry
The environmental movement that paved the way for green chemistry began in 1962 with the publication of Rachel Carson's "Silent Spring," which highlighted the adverse effects of chemicals on the environment [111]. The establishment of the Environmental Protection Agency (EPA) in the United States in the 1970s marked a significant step toward environmental protection, followed by key international developments including the 1972 Stockholm Conference and the formation of the World Commission on Environment and Development in 1983, which introduced the concept of sustainable development [111]. The formal foundations of green chemistry were laid in the early 1990s by Paul Anastas and John C. Warner, scientists at the EPA [111]. The introduction of the annual Presidential Green Chemistry Challenge Awards in 1996 helped draw attention to both academic and industrial success stories in the field [16].
Green chemistry served as the guiding philosophy to instigate clean products and processes that eliminate the use or generation of hazardous substances [110] [111]. Its primary objective has been to promote sustainability and conserve natural resources by preventing the release of harmful chemicals into the environment, designing cost-effective and less hazardous chemical synthesis, and fostering alternative technologies with minimal impact on human health and ecosystems [111]. The approach is fundamentally based on the aphorism "an ounce of prevention is worth a pound of cure," focusing on minimizing hazard through molecular design rather than managing risk through exposure controls [16].
The Emergence of Circular Chemistry
Circular chemistry represents a more recent evolution in sustainable chemical thinking, combining concepts from green chemistry, circular economy, and sustainability into a new conceptual framework [110]. While green chemistry principles align more closely with linear economy approaches, circular chemistry specifically aims to transform the chemical industry into a closed-loop and waste-free system [110]. This transition is increasingly urgent given that current consumption and production patterns continue to create unsustainable pressures on the environment, with the extraction and processing of resources accounting for half of total greenhouse gas emissions and more than 90% of biodiversity loss and water stress impacts [110].
The framework for circular chemistry has been articulated through twelve goals that facilitate the transition to a resource-efficient, closed-loop, and waste-free analytical chemistry sector [110]. Unlike green chemistry, which primarily focuses on laboratory practices and the environmental impact of consumption and disposal phases, circular chemistry targets the radical transformation of the entire chemical system of production, consumption, and waste by connecting post-use and production while preserving natural resources, environment, and human health [110]. It emphasizes keeping materials in circulation for as long as possible and relies on changes made by a broad alliance of stakeholders including academia, industries, governments, and organizations [110].
Table 1: Historical Evolution of Sustainable Chemistry Frameworks
| Time Period | Key Developments | Primary Focus |
|---|---|---|
| 1960s-1970s | Publication of "Silent Spring" (1962), EPA establishment (1970), Stockholm Conference (1972) | Environmental awareness, pollution control |
| 1980s-1990s | Concept of sustainable development (1983), Pollution Prevention Act (1990), 12 Principles of Green Chemistry (1998) | Waste prevention, hazard reduction, atom economy |
| 2000s-2010s | Green Chemistry journal launch (1999), International symposia, Green Nano concept | Solvent alternatives, renewable feedstocks, energy efficiency |
| 2020s-Present | Circular chemistry framework, Global Framework on Chemicals (2023), Sustainable Development Goals integration | Closed-loop systems, resource efficiency, multi-stakeholder collaboration |
Comparative Analysis of Core Principles and Goals
The Twelve Principles of Green Chemistry
The foundational framework of green chemistry is organized around twelve principles that provide comprehensive guidelines for designing chemical products and processes with reduced environmental impact [111]. These principles emphasize:
- Waste prevention rather than cleanup after generation
- Atom economy in synthetic processes
- Less hazardous chemical syntheses
- Designing safer chemicals with reduced toxicity
- Safer solvents and auxiliaries
- Design for energy efficiency
- Use of renewable feedstocks
- Reduced derivatives in synthetic pathways
- Catalysis over stoichiometric reagents
- Design for degradation after use
- Real-time analysis for pollution prevention
- Inherently safer chemistry for accident prevention [111]
These principles primarily focus on the molecular level and individual processes, aiming to minimize environmental impact through improved design. For example, the principle of atom economy refers to the utilization of atoms from starting materials with maximum efficiency in the final product, where ideal reactions incorporate all atoms of the reactants [111]. The Diels-Alder reaction is often cited as an exemplary green reaction with theoretical 100% atom economy since all atoms from the reactants are incorporated into the final product [111].
The Twelve Goals of Circular Chemistry
Circular chemistry expands beyond the green chemistry principles with twelve goals that encompass the entire life cycle of chemical products [110]. These goals include:
- Waste-free and resource-efficient analysis
- Circulation of materials and products
- Minimization of hazards
- Renewable energy integration
- Process intensification and miniaturization
- Digitalization and smart technologies
- Standardization and harmonization
- Economic sustainability
- Education and knowledge sharing
- Collaboration and partnerships
- Policy and regulatory support
- Global perspective and equitable access [110]
The circular chemistry framework aims to decouple analytical performance from resource consumption rates and requires a strong alliance of all stakeholders to transform the entire system of production, consumption, and waste [110]. Unlike green chemistry, which focuses primarily on environmental aspects, circular chemistry explicitly integrates economic considerations and acknowledges the need for global equity in resource access and technology deployment [110].
Table 2: Comparative Analysis of Green Chemistry Principles vs. Circular Chemistry Goals
| Aspect | Green Chemistry | Circular Chemistry |
|---|---|---|
| Primary Focus | Molecular design, hazard reduction | System transformation, resource circulation |
| Economic Model | Aligned with linear economy | Circular economy implementation |
| Time Perspective | Focus on design and production stages | Full life cycle perspective |
| Stakeholder Engagement | Primarily chemists and manufacturers | Multi-stakeholder collaboration |
| Energy Strategy | Energy efficiency | Renewable energy integration |
| Waste Management | Waste minimization | Waste elimination through circulation |
| Scale of Implementation | Process and product level | System and sector level |
Methodological Approaches and Experimental Protocols
Green Chemistry Methodologies in Practice
Green chemistry has developed numerous methodological approaches that align with its twelve principles. In pharmaceutical development and analytical chemistry, these include:
Solvent-Free Methodologies: Implementation of solvent-free extraction techniques that eliminate the need for organic solvents, reducing toxicity and waste generation [111]. These methods often employ mechanical activation, microwave assistance, or supercritical fluid extraction to enhance efficiency without solvent use.
Catalytic System Optimization: Development of selective catalysts to replace stoichiometric reagents, as demonstrated in the synthesis of niobium-based catalysts for biomass valorization [112]. For example, researchers have developed niobium oxide nanoparticles embedded in mesoporous silica matrices that show high stability in recycling runs for reactions such as aldol condensation of furfural with acetone and esterification reactions of biomass-derived acids [112].
Green Synthesis of Nanoparticles: Utilization of plant-derived biomolecules as reducing and stabilizing agents in the synthesis of silver nanoparticles (AgNPs) [111]. These eco-friendly approaches eliminate hazardous chemicals while yielding biocompatible nanoparticles with enhanced antimicrobial and catalytic properties, demonstrating potential in nanotechnology and biomedical applications [111].
Green Sample Preparation (GSP): Implementation of the ten principles of GSP that focus on minimizing materials and energy input while maximizing sample throughput through acceleration, parallel processing, automation, and step integration [113]. Effective approaches include applying vortex mixing or assisting fields such as ultrasound and microwaves to enhance extraction efficiency while consuming significantly less energy compared to traditional methods like Soxhlet extraction [113].
Circular Chemistry Implementation Frameworks
Circular chemistry requires more comprehensive methodological approaches that address the entire life cycle of chemical products:
Resource Recovery and Recycling: Development of closed-loop recycling systems for chemical products and materials. Examples include hydrometallurgical recycling technologies for NMC Li-ion battery cathodes that recover critical materials like lithium, cobalt, and nickel [114], and chemical recycling of PET to value-added products through depolymerization strategies that transform plastic waste into fine chemicals and monomers [114].
Industrial Symbiosis Integration: Implementation of systems where waste streams from one process become feedstocks for another, as demonstrated in flexible large-scale environmentally sustainable methanol and ammonia co-production using industrial symbiosis that integrates green hydrogen and carbon capture [114].
Biomass Valorization Pathways: Transformation of agricultural residues and other waste biomass into valuable chemical feedstocks, such as the conversion of furfural (an industrial platform chemical derived from carbohydrates) to drop-in fuels using relatively cheap and green catalysts [112]. These approaches contribute to a greener world by developing sustainable alternatives to fossil fuels using renewable and cheap energy sources [112].
Safe and Sustainable by Design (SSbD) Principles: Integration of safety and sustainability considerations early in the design process of chemicals and materials, incorporating toxicity and hazard screening, environmental footprint assessments, and systems thinking to scale molecular-level inventions into safe and sustainable societal advancements [115].
Diagram 1: Circular Chemistry Workflow for Biomass Valorization. This diagram illustrates the integrated process for converting biomass into value-added products while maintaining material circulation and renewable energy integration.
Research Reagent Solutions and Essential Materials
The implementation of both green and circular chemistry principles requires specific research reagents and materials that enable sustainable chemical processes. The following table details key solutions used in advanced sustainable chemistry applications:
Table 3: Essential Research Reagents for Sustainable Chemistry Applications
| Reagent/Material | Function | Application Example | Sustainability Benefit |
|---|---|---|---|
| Niobium-based catalysts | Acidic catalyst for biomass conversion | Valorization of furfural to fuel precursors | Water-tolerant, stable in recycling runs, replaces hazardous acids [112] |
| Dipyridyldithiocarbonate (DPDTC) | Environmentally responsible reagent | Synthesis of esters and thioesters | Enables solvent-free or green solvent reactions, by-product recycling [112] |
| Bio-based solvents | Green reaction media | Replacement for volatile organic compounds | Renewable feedstocks, reduced toxicity and emissions [111] |
| Ionic liquids | Designer solvents for selective extraction | Processing of biomass and waste streams | Low volatility, tunable properties, recyclability [111] |
| Enzymatic catalysts | Biocatalysis for selective transformations | Pharmaceutical intermediates synthesis | Biodegradable, high selectivity, mild reaction conditions [111] |
| Mesoporous silica supports | Catalyst support material | Embedded nanoparticle catalysts | Enhanced stability, recyclability, controlled reactivity [112] |
| Supercritical fluids | Solvent and reaction medium | Extraction and chemical reactions | Tunable properties, easily separated from products [111] |
Metrics and Assessment Frameworks
Green Chemistry Metrics
The evaluation of green chemistry implementations relies on specific metrics that assess environmental performance:
Atom Economy: Calculation of the efficiency of incorporating starting materials into the final product [111]. This metric is calculated as (molecular weight of desired product / molecular weight of all reactants) × 100%.
E-factor: Measurement of waste generation per unit of product, calculated as total waste mass divided by product mass [111].
Life Cycle Assessment (LCA): Comprehensive evaluation of environmental impacts across the entire life cycle of a product or process [116].
Greenness Assessment Tools: Standardized metrics like the AGREEprep tool that provide quantitative scores for method greenness based on multiple criteria [113]. Recent assessments of 174 standard methods from CEN, ISO, and Pharmacopoeias revealed that 67% scored below 0.2 on the AGREEprep scale (where 1 represents the highest possible score), highlighting the need for method updating [113].
Circular Chemistry Indicators
Circular chemistry requires broader assessment frameworks that capture system-level circularity:
Material Circularity Indicator (MCI): Measurement of how effectively materials are circulated in closed loops [114].
Renewable Resource Integration: Assessment of the proportion of renewable versus finite resources in chemical processes [110].
Resource Efficiency Metrics: Evaluation of resource productivity (economic output per unit of resource input) and circulation rates [114]. Currently, only about 7.2% of materials are cycled globally, a decline from 9.1% in 2018, highlighting the urgency for improved circularity [114].
Sustainable Chemistry Indicators: Development of comprehensive indicator sets for international chemicals management, comprising 23 indicators based on defined criteria that consider interfaces with global resource management, health protection, climate protection, circular economy, and biodiversity [116].
Diagram 2: Integrated Assessment Framework for Sustainable Chemistry. This diagram shows the relationship between different chemistry frameworks and their corresponding evaluation metrics across technical, environmental, economic, and social dimensions.
Implementation Challenges and Future Directions
Barriers to Adoption
The transition from traditional chemical practices to green and circular chemistry faces several significant challenges:
Coordination Failure: Limited cooperation between key stakeholders like industry and academia makes it difficult to transition to circular processes that require far more cooperation than conventional linear methods [113]. Analytical chemistry remains a traditional and conservative field, with disconnected key players hindering the collaboration needed for circularity [113].
Regulatory Inertia: Outdated standard methods persist in official methodologies, with greenness assessments revealing that 67% of standard methods score below 0.2 on the AGREEprep scale [113]. Regulatory agencies have been slow to integrate green metrics into method validation and approval processes [113].
Economic Barriers: Many promising sustainable processes face high costs that hinder implementation. As noted in green chemistry research, "sustainable processes for producing biofuels and biobased chemicals continues to demand much investigation" due to economic challenges [112].
Rebound Effects: In green analytical chemistry, the rebound effect occurs when efforts to reduce environmental impact lead to unintended consequences that offset the intended benefits [113]. For example, novel low-cost microextraction methods might lead laboratories to perform significantly more extractions than before, increasing the total volume of chemicals used and waste generated [113].
Future Research and Development Priorities
The future advancement of sustainable chemistry requires focused efforts in several key areas:
Integration of Artificial Intelligence: AI and machine learning applications for optimizing material synthesis and improving efficiency in chemical processes [111]. AI-driven approaches can rapidly identify and design new sustainable catalysts and reaction pathways, minimizing waste and energy consumption [111].
Advanced Biomass Valorization: Development of more efficient processes for converting complex biobased feedstocks, proteins, biomass, and CO₂ into sustainable building blocks [115]. This includes refining biorefineries based on novel feedstocks and advancing green catalytic synthesis pathways [115].
Safe and Sustainable by Design (SSbD) Frameworks: Enhanced integration of SSbD principles that incorporate toxicity and hazard screening, environmental footprint assessments, and systems thinking into chemical development [61] [115]. This includes developing New Approach Methodologies (NAMs) for early hazard identification and predictive toxicology approaches [115].
Circular Economy Implementation: Accelerating the transition from linear to circular models through improved recycling technologies, material design for circularity, and business models that support chemical leasing and service-based approaches [110] [114]. This is increasingly urgent given that global material consumption has surged, with over 500 billion tonnes used in the past five years—nearly equal to total consumption during the entire 20th century [114].
The evolution from green chemistry to circular chemistry represents a necessary paradigm shift in the chemical sciences, expanding the focus from molecular-level hazard reduction to system-wide resource efficiency and material circulation. While green chemistry has provided essential foundational principles for reducing waste and toxicity in chemical processes, circular chemistry offers a more comprehensive framework for addressing the full life cycle of chemical products and decoupling chemical production from resource consumption. The integration of these approaches, along with emerging Safe and Sustainable by Design (SSbD) methodologies, presents the most promising pathway for achieving a truly sustainable chemical industry [61].
For researchers, scientists, and drug development professionals, understanding the distinctions and complementarities between these frameworks is crucial for navigating the transition toward sustainable chemistry practices. The successful implementation of these approaches will require unprecedented collaboration across academia, industry, governments, and organizations—breaking down traditional silos and building bridges to accelerate the shift toward a waste-free and resource-efficient sector [110] [113]. As the field continues to evolve, the integration of artificial intelligence, advanced biomass valorization techniques, and comprehensive sustainability metrics will play increasingly important roles in driving innovation and measuring progress toward a circular chemical economy that supports both human needs and planetary health.
The sustainable chemistry movement has evolved from a framework of principles into a discipline driven by technological innovation. Rooted in foundational concepts like the 12 principles of Green Chemistry established in the 1990s, the field initially focused on minimizing hazardous waste and reducing the use of toxic solvents [15]. This philosophy has progressively shifted from pollution prevention and risk reduction to a systems-level approach that integrates advanced technologies to address environmental, economic, and societal impacts simultaneously [62]. This evolution marks a transition from intuition-driven and theory-driven research to a new paradigm characterized by the deep integration of data-driven approaches and physical insights [117].
Within this new paradigm, two frontier innovations are poised to redefine the capabilities of chemical research and manufacturing: AI-guided catalysis and bio-based polymers. The former leverages artificial intelligence to accelerate the discovery and optimization of catalytic processes, a critical component in making chemical reactions more efficient and sustainable. The latter represents a shift in material sourcing, moving from finite fossil resources to renewable biomass. Both innovations are intrinsically linked; the development of efficient, sustainable catalysts is often essential for the economically viable production of advanced bio-based polymers. This in-depth technical guide examines the state of these technologies, their convergence, and the practical methodologies enabling their advancement within the modern research laboratory.
The Rise of AI-Guided Catalysis
A Paradigm Shift in Catalyst Discovery and Optimization
Catalysis stands as a cornerstone of modern chemical processes, playing a pivotal role in everything from pharmaceutical synthesis to energy production. Traditional catalyst discovery, however, has been limited by reliance on trial-and-error experimentation and computationally intensive theoretical simulations, which struggle to navigate the vastness of chemical space [117] [118]. Artificial Intelligence, particularly Machine Learning (ML), has emerged as a transformative tool that breaks these constraints.
ML excels at extracting patterns from complex, multidimensional datasets to make accurate predictions. In catalysis, its application follows a hierarchical framework progressing from data-driven screening to performance modeling with physical descriptors, and ultimately to symbolic regression aimed at uncovering general catalytic principles [117]. This approach has transformed ML from a mere predictive tool into a "theoretical engine" that contributes to mechanistic discovery [117].
Table 1: Key Machine Learning Paradigms in Catalysis Research
| Learning Paradigm | Data Type | Primary Applications in Catalysis | Key Advantages |
|---|---|---|---|
| Supervised Learning | Labeled Data | Predicting reaction yield, selectivity, and catalytic activity [118]. | High predictive accuracy for well-defined tasks [118]. |
| Unsupervised Learning | Unlabeled Data | Clustering catalysts/ligands by similarity; dimensionality reduction for data visualization [118]. | Reveals hidden patterns and structures without pre-existing labels [118]. |
| Reinforcement Learning | Interaction with Environment | Optimizing catalyst performance through iterative virtual testing and closed-loop systems [119]. | Enables autonomous discovery and optimization [119]. |
Core Machine Learning Algorithms for Catalysis
Several ML algorithms have proven particularly effective for chemical applications. The choice of algorithm depends on the nature of the data and the specific problem, such as predicting a continuous value (regression) or a category (classification).
- Linear Regression: A fundamental model that establishes a linear relationship between molecular descriptors and catalytic outcomes. It can serve as a powerful baseline; for instance, Multiple Linear Regression (MLR) has been used to model activation energies for Pd-catalyzed allylation with high accuracy (R² = 0.93), successfully quantifying electronic and steric effects [118].
- Random Forest: An ensemble method that constructs multiple decision trees during training and outputs the average prediction (regression) or the modal class (classification). This algorithm is robust against overfitting and can handle high-dimensional descriptor spaces, making it ideal for predicting catalytic performance from hundreds of molecular features [118].
- Neural Networks/Deep Learning: These multi-layer networks model complex, non-linear relationships between structure and performance. They are particularly effective with large, diverse datasets and are the foundation for more advanced generative AI models that can suggest entirely new molecular structures [118] [119].
Experimental Protocol: An Interpretable ML Workflow for Catalyst Design
The following detailed methodology outlines the process for designing earth-abundant catalysts for ammonia cracking, a reaction critical for clean hydrogen production [120].
1. Problem Definition and Data Acquisition:
- Objective: Identify earth-abundant, low-cost catalysts for efficient ammonia (NH₃) decomposition under non-thermal plasma (NTP) conditions to replace scarce ruthenium (Ru).
- Data Collection: Curate a dataset of catalytic performance metrics (e.g., conversion rate, turnover frequency) for various catalysts from high-throughput experiments or computational simulations (e.g., Density Functional Theory). The initial study screened over 3,300 alloy compositions [120].
2. Multi-Scale Simulation and Descriptor Identification:
- Employ multi-scale simulations spanning atomic-to-reactor scales to model catalyst behavior under plasma conditions.
- Identify key physical descriptors governing activity. For NH₃ decomposition, nitrogen adsorption energy (EN) was identified as a critical descriptor. Under plasma, the optimal EN shifts from -0.9 eV (favoring Ru) to -0.5 eV, placing inexpensive cobalt (Co) at the top of the activity volcano plot [120].
3. Model Training and Interpretation:
- Train interpretable ML models (e.g., Random Forest with SHAP analysis) to predict catalytic activity based on the identified descriptors and alloy features.
- Use SHAP (Shapley Additive Explanations) analysis to reveal the underlying physical factors driving performance. In the case of bimetallic catalysts, SHAP consistently highlighted d-band filling as the dominant electronic descriptor, aligning with fundamental catalysis theory [120].
4. Validation and Techno-Economic Assessment (TEA):
- Synthesize and experimentally validate the top-performing catalyst candidates identified by the ML screen (e.g., Ni₃Mo, Fe₃Cu).
- Conduct TEA and Life Cycle Assessment (LCA) to evaluate commercial viability. The study showed Ni₃Mo could deliver H₂ at costs below $1 per kilogram with a carbon footprint of ~0.91 kg CO₂ per kg H₂ [120].
The Scientist's Toolkit: Key Reagents and Materials for AI-Guided Catalysis
Table 2: Essential Research Reagents and Computational Tools for AI-Guided Catalysis
| Item | Function/Description | Example in Use |
|---|---|---|
| Density Functional Theory (DFT) | Computational method to calculate electronic structure properties of molecules and solids. | Used to generate training data, e.g., calculating nitrogen adsorption energies (E_N) for thousands of catalyst surfaces [120]. |
| Molecular Descriptors | Quantifiable properties representing steric, electronic, and structural features of a catalyst or ligand. | Used as model inputs; can include steric volume parameters, electronic parameters (d-band center), and topological indices [117] [118]. |
| SHAP (SHapley Additive exPlanations) | A game theory-based method to explain the output of any ML model. | Provides interpretability by revealing the contribution of each descriptor (e.g., d-band filling) to the predicted catalytic activity [120]. |
| Non-Thermal Plasma (NTP) Reactor | A reactor generating a plasma field to create reactive species, enabling reactions under milder conditions. | Key experimental setup for validating ML-predicted catalysts for ammonia cracking, allowing operation at lower temperatures [120]. |
| High-Throughput Experimentation (HTE) | Automated platforms for rapidly testing thousands of chemical reactions in parallel. | Generates large, standardized datasets required for training robust ML models on experimental (not just computational) data [117]. |
The Expansion of Bio-Based Polymers
From Niche to Mainstream: Market and Material Evolution
Bio-based polymers are materials produced from renewable resources, offering a sustainable alternative to petroleum-derived plastics. It is critical to distinguish between bio-based (origin of carbon) and biodegradable (end-of-life behavior); a polymer can be one, both, or neither [121]. While currently representing a small fraction (~1%) of the global polymer market, their production is projected to grow at a Compound Annual Growth Rate (CAGR) of 13-15% through 2035, substantially outpacing conventional polymers. By 2035, bio-based polymers could capture 4-5% of global production, equating to 25-30 million tonnes annually [122].
This growth is fueled by corporate sustainability agendas, regulatory frameworks, and technological breakthroughs. The market is segmented into biodegradable (e.g., Polylactic Acid - PLA, Polyhydroxyalkanoates - PHA) and non-biodegradable (e.g., bio-based Polyethylene - PE, Polyamide - PA) polymers, each with distinct growth dynamics and applications [122].
Table 3: Key Bio-Based Polymers, Properties, and Commercial Status
| Polymer Type | Feedstock | Key Properties | Example Applications | Production Capacity & Trends |
|---|---|---|---|---|
| Polylactic Acid (PLA) | Corn, Sugars | Good transparency, high rigidity, compostable. | Food packaging, textiles, 3D printing filaments, medical devices [121]. | Dominated by NatureWorks (Ingeo). Global capacity expanding rapidly, ~140 kton/year [121]. |
| Bio-based PE & PP | Sugarcane-derived ethanol | Identical properties to fossil-based counterparts; non-biodegradable. | Flexible packaging, cosmetics, hygiene products, automotive parts [123] [122]. | Braskem is a major producer. High growth potential, particularly for PP [123] [122]. |
| Polyhydroxyalkanoates (PHA) | Microbial fermentation from sugars/lipids | Biodegradable in various environments, biocompatible. | Bioactive medical devices, compostable packaging, agricultural films [121] [122]. | Emerging polymer with high growth potential (CAGR ~17%); produced by Danimer Scientific, RWDC Industries [122]. |
| Bio-based Polyethylene Furanoate (PEF) | Sugar-derived FDCA | Superior barrier properties (O₂, CO₂) vs. PET. | Beverage bottles, food packaging. | Emerging polymer; Avantium is a key developer. Poised for substantial market entry [122]. |
Experimental Focus: Enhancing PLA for High-Performance Applications
Polylactic Acid (PLA) is a leading bio-based polyester, but its low glass transition temperature (~60°C) limits use in high-temperature applications. The following protocol details methodologies for enhancing its thermal and mechanical properties.
1. Controlling Stereochemistry:
- Method: Synthesize PLA with varying ratios of L- and D-lactic acid enantiomers. This is achieved during the ring-opening polymerization of lactide by controlling the catalyst and monomer feed.
- Rationale: The ratio of L/D isomers directly influences the crystallinity and melting temperature (Tm) of the final polymer. A higher L-content leads to higher crystallinity and Tm [121].
- Data: A 100:0 L/D-PLA has a Tm of 178°C, while an 80:20 blend has a Tm of 125°C [121].
2. Polymer Blending and Compounding:
- Method: Melt-blend PLA with other polymers or additives to create composite materials.
- Protocol:
- Pre-drying: Dry PLA pellets and additive masterbatch in a vacuum oven at 80°C for 4 hours to prevent hydrolysis.
- Melt Processing: Use a twin-screw extruder with a temperature profile from the feed zone (180°C) to the die (210°C).
- Additives: Incorporate impact modifiers, nucleating agents, or natural fibers (e.g., kenaf) to improve toughness and heat resistance.
- Validation: Companies like NEC and Fujitsu have developed PLA composites with carbon/kenaf fibers or polycarbonate blends for use in electronics housings, significantly improving thermal and flame-retardant properties [121].
3. Advanced Processing with Machine Direction Orientation (MDO):
- Method: Post-process PLA films using MDO technology, which stretches the film in the machine direction to orient the polymer chains.
- Outcome: This enhances stiffness, optical clarity, and barrier properties, enabling the creation of high-performance mono-material packaging (e.g., stand-up pouches) that are fully recyclable and align with the circular economy [123].
Concluding Perspective: The Converging Frontier
The frontiers of AI-guided catalysis and bio-based polymers are not isolated trajectories; they are increasingly interdependent. The efficient, sustainable production of advanced bio-based monomers like FDCA for PEF or lactide for PLA relies on the development of highly active and selective catalysts. AI-guided catalyst design is poised to dramatically accelerate the discovery of these tailored catalysts, thereby enabling the next generation of bio-based materials with improved properties and lower costs.
This convergence exemplifies the necessary evolution of green chemistry into a systems-based, interdisciplinary practice of sustainable chemistry [62]. Success in this new era requires chemists and material scientists to integrate life cycle assessment, techno-economic analysis, and advanced data science with molecular design. By embracing this holistic, data-driven approach, researchers can truly validate and deliver on the promise of these frontier innovations, transforming the chemical industry and contributing to a more sustainable future.
Conclusion
The historical journey of green chemistry demonstrates a fundamental paradigm shift from pollution control to prevention, establishing a robust framework that is both scientifically sound and ethically imperative. The synthesis of foundational principles, practical methodologies, strategic optimization, and validated successes confirms that sustainable chemistry is not a constraint but a powerful engine for innovation in drug development. For biomedical and clinical research, the future direction is clear: deeper integration of green chemistry with circular economy models and Safe-and-Sustainable-by-Design (SSbD) principles. This will be accelerated by digital tools like AI, which will enable the predictive design of safer molecules and more efficient processes. Embracing this holistic approach is essential for developing the next generation of therapeutics in a way that ensures economic viability and minimizes environmental impact, ultimately contributing to a more resilient and sustainable healthcare ecosystem.
References
- 1. End-Of-Pipe Solutions → Term [https://pollution.sustainability-directory.com/term/end-of-pipe-solutions/]
- 2. Timeline of Environmental Movement and History [https://www.pbs.org/wnet/americanmasters/a-fierce-green-fire-timeline-of-environmental-movement/2988/]
- 3. Environmental movement in the United States [https://en.wikipedia.org/wiki/Environmental_movement_in_the_United_States]
- 4. Cleantech vs. End-of-Pipe: Choosing Sustainable ... [https://incit.org/en_us/thought-leadership/cleantech-or-end-of-pipe-which-should-manufacturers-choose/]
- 5. Basics of Green Chemistry [https://www.epa.gov/greenchemistry/basics-green-chemistry]
- 6. Legacy of Rachel Carsons Silent Spring National Historic ... [https://www.acs.org/education/whatischemistry/landmarks/rachel-carson-silent-spring.html]
- 7. Silent Spring [https://en.wikipedia.org/wiki/Silent_Spring]
- 8. Silent Spring and the Modern Environmental Movement [https://www.tamucc.edu/library/exhibits/s/sts/page/silent-spring]
- 9. The History of Green Chemistry [https://www.acs.org/green-chemistry-sustainability/what-is-green-chemistry/history-of-green-chemistry1.html]
- 10. History of Green Chemistry [https://chemistryforsustainability.org/history-green-chemistry]
- 11. The Love Canal Tragedy | About EPA [https://www.epa.gov/archive/epa/aboutepa/love-canal-tragedy.html]
- 12. Love Canal [https://en.wikipedia.org/wiki/Love_Canal]
- 13. Pollution Prevention Act of 1990 [https://www.epa.gov/p2/pollution-prevention-act-1990]
- 14. Pollution Prevention Act of 1990 [https://en.wikipedia.org/wiki/Pollution_Prevention_Act_of_1990]
- 15. Evolution of green chemistry and its multidimensional ... [https://www.sciencedirect.com/science/article/pii/S131901641830152X]
- 16. History of Green Chemistry [https://greenchemistry.yale.edu/about/history-green-chemistry]
- 17. The Origins of EPA | US EPA [https://www.epa.gov/history/origins-epa]
- 18. Evolution of green chemistry and its multidimensional impacts [https://pmc.ncbi.nlm.nih.gov/articles/PMC6323129/]
- 19. TRI Green Chemistry and Green Engineering Reporting [https://www.epa.gov/toxics-release-inventory-tri-program/tri-green-chemistry-and-green-engineering-reporting]
- 20. Chemical Safety for Sustainability Strategic Research ... [https://www.epa.gov/research/chemical-safety-sustainability-strategic-research-action-plan-2019-2022]
- 21. Green Chemistry: A More Sustainable Approach to ... [https://www.pfizer.com/news/articles/green_chemistry_a_more_sustainable_approach_to_medicine_development]
- 22. Harnessing the 12 Green Chemistry Principles for Sustainable ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11184551/]
- 23. 12 Principles of Green Chemistry [https://www.acs.org/green-chemistry-sustainability/principles/12-principles-of-green-chemistry.html]
- 24. Twelve Principles of Green Chemistry – Cooperative Organic Chemistry Student Laboratory Manual [https://openbooks.lib.msu.edu/orgchemlabmanual/chapter/twelve-principles-of-green-chemistry/]
- 25. Sustainable drug discovery using Green Chemistry [https://www.astrazeneca.com/content/astraz/what-science-can-do/topics/sustainability/sustainable-drug-discovery-using-green-chemistry.html]
- 26. Sustainable chemistry challenges from a developing ... [https://www.sciencedirect.com/science/article/abs/pii/S2452223617300524]
- 27. Green chemistry and responsible research and innovation [https://www.sciencedirect.com/science/article/pii/S0959652624034607]
- 28. Comment: Addressing challenges to advance sustainable ... [https://cen.acs.org/acs-news/comment/Comment-Addressing-challenges-advance-sustainable/102/i12]
- 29. Green Chemistry History - American Chemical Society [https://www.acs.org/green-chemistry-sustainability/what-is-green-chemistry/history-of-green-chemistry.html]
- 30. ACS GCI Pharmaceutical Roundtable Reagent Guides [https://reagents.acsgcipr.org/]
- 31. Green Chemistry Challenge Winners [https://www.epa.gov/greenchemistry/green-chemistry-challenge-winners]
- 32. Future Origins Receives National Award for Green ... [https://www.futureorigins.com/articles/future-origins-receives-national-award-for-green-chemistry-from-acs]
- 33. 2025 Green Chemistry Challenge Award Winners [https://www.acs.org/green-chemistry-sustainability/funding-and-recognition/gcca/2025-awards.html]
- 34. a systematic review of early phase sustainability assessments ... [https://pubs.rsc.org/en/content/articlehtml/2025/gc/d5gc02565f]
- 35. Spotlight on Emily Rouse: Advancing Solvent-Free ... [https://www.vanderbilt.edu/vinse/2025/08/19/spotlight-on-emily-rouse-advancing-solvent-free-organometallic-synthesis-and-inspiring-future-scientists-through-outreach/]
- 36. Mechanochemistry-driven solvent-free synthesis of ... [https://pubs.rsc.org/en/content/articlehtml/2025/mr/d5mr00068h]
- 37. DOZNTM3.0: A Quantitative Green Chemistry Evaluator for ... [https://www.sciencedirect.com/science/article/pii/S2772826925000744]
- 38. Water as the reaction medium in organic chemistry [https://pubs.rsc.org/en/content/articlehtml/2021/sc/d0sc06000c]
- 39. How water could replace some organic solvents and make ... [https://www.cas.org/resources/cas-insights/organic-solvents]
- 40. A Comprehensive Review on Deep Eutectic Solvents and Its ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10820243/]
- 41. The environmental origin and fate of deep eutectic solvents [https://www.sciencedirect.com/science/article/pii/S0167732225021051?dgcid=rss_sd_all]
- 42. Editorial: Advances in the development and application of ... [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2023.1258718/full]
- 43. Application of deep eutectic solvents for the determination ... [https://www.sciencedirect.com/science/article/pii/S2772582025000117]
- 44. Optimizing saffron bioactive extraction with deep eutectic ... [https://www.nature.com/articles/s41598-025-99695-1]
- 45. Application and Efficiency of Deep Eutectic Solvents in the ... [https://pubmed.ncbi.nlm.nih.gov/40610205/]
- 46. Responsive deep eutectic solvents: mechanisms ... [https://pubs.rsc.org/en/content/articlelanding/2025/cc/d4cc05157b]
- 47. Closing the Loop in Pharma and Biopharma Production [https://www.pharmasalmanac.com/articles/circular-biomanufacturing-closing-the-loop-in-pharma-and-biopharma-production]
- 48. Top 10 Carbon Reduction Strategies for ... [https://www.pharmafocusamerica.com/articles/top-10-carbon-reduction-strategies-pharma-2025]
- 49. Harnessing Nature's Bounty: Renewable Feedstocks in ... [https://pharmafeatures.com/harnessing-natures-bounty-renewable-feedstocks-in-modern-chemical-processes/]
- 50. Making natural products from renewable feedstocks [https://pubs.rsc.org/en/content/articlehtml/2020/np/c9np00040b]
- 51. Biobased, Recycled and Renewable Carbon Chemicals [https://www.resourcewise.com/blog/chemicals-blog/biobased-recycled-and-renewable-carbon-chemicals-an-introduction]
- 52. Green chemistry: Six key trends to watch [https://www.cas.org/resources/cas-insights/green-chemistry-trends]
- 53. Sustainable Chemistry 2025: Trends Transforming Industry [https://www.custommarketinsights.com/chemical-and-materials/sustainable-chemistry-2025/]
- 54. Sustaining Sustainable Chemistry [https://issues.org/sustainable-green-chemistry-tickner-rubin-shen-maxwell-jones-kirchoff/]
- 55. AI in Predictive Toxicology Market Outlook 2025–2032 [https://finance.yahoo.com/news/ai-predictive-toxicology-market-outlook-150800478.html]
- 56. AI in Predictive Toxicology Market Forecast, 2025-2032 [https://www.coherentmarketinsights.com/industry-reports/ai-in-predictive-toxicology-market]
- 57. Transformative Role of Artificial Intelligence in Drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12406033/]
- 58. AI-based toxicity prediction models using ToxCast data [https://www.sciencedirect.com/science/article/pii/S0300483X25001891]
- 59. New Catalyst Design Solves a Decades-Old Chemical ... [https://scitechdaily.com/new-catalyst-design-solves-a-decades-old-chemical-challenge/]
- 60. Sustainable Reaction Engineering [https://www.ceb.cam.ac.uk/research/groups/rg-sre]
- 61. Sustainable chemistry: Green, circular, and safe-by-design [https://www.sciencedirect.com/science/article/pii/S2590332224001969]
- 62. Green and sustainable chemistry – The case for a systems ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8654969/]
- 63. Green Chemistry: The Role of Enzymes in Sustainable ... [https://www.amano-enzyme.com/news/green-chemistry-the-role-of-enzymes-in-sustainable-solutions/]
- 64. Atom Economy Green Synthesis in Organic Chemistry [https://www.jocpr.com/articles/atom-economy-green-synthesis-in-organic-chemistry-10314.html]
- 65. Industrial Catalyst Market Faces Shift Toward Efficiency, ... [https://breakthroughgroup.com/market_watch/industrial-catalyst-market-growth-trends-forecast-2025-top/]
- 66. Sustainable Catalytic Strategies for Carbon ... [https://www.sciencedirect.com/science/article/abs/pii/S0926860X25006167]
- 67. Progress in heterogeneous catalysis for renewable energy ... [https://www.sciencedirect.com/science/article/pii/S0378382025000918]
- 68. The Role of Industrial Catalysts in Accelerating ... [https://www.mdpi.com/2673-4583/17/1/6]
- 69. Green innovations in C–H bond functionalisation [https://pubs.rsc.org/en/content/articlehtml/2025/gc/d5gc00278h]
- 70. Global Scientists Issue Nobel Declaration Calling for Urgent ... [https://environment.yale.edu/news/article/nobel-declaration-calling-urgent-implementation-green-sustainable-chemistry]
- 71. Waking up to the costs of green technology adoption ... [https://link.springer.com/article/10.1007/s43621-025-01239-0]
- 72. Green technology adoption under uncertainty, increasing ... [https://www.sciencedirect.com/science/article/pii/S0167268125000733]
- 73. Revealing drivers of green technology adoption through ... [https://www.sciencedirect.com/science/article/pii/S2666792425000368]
- 74. Challenges Moeve light up - Green Hydrogen Innovation [https://www.moeveglobal.com/en/innovation/moeve-light-up/moeve-light-up-challenges]
- 75. Optimizing sustainability performance through digital ... [https://www.nature.com/articles/s41598-025-04912-6]
- 76. An Overview of Potential Alternatives for the Multiple Uses ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11800378/]
- 77. Emerging PFAS alternatives: Unveiling environmental fates ... [https://www.sciencedirect.com/science/article/pii/S3050745625000409]
- 78. EPA Sharpens the Focus of Its PFAS Regulatory Framework ... [https://pfas.pillsburylaw.com/epa-pfas-regulatory-framework-october-2025-update/]
- 79. EPA's PFAS Rulemaking Trajectory: Key Updates Across ... [https://www.hklaw.com/en/insights/publications/2025/10/epas-pfas-rulemaking-trajectory-key-updates]
- 80. PFAS could be replaced with safe graphene oxide solution [https://news.northwestern.edu/stories/2025/05/pfas-could-be-replaced-with-safe-graphene-oxide-solution]
- 81. Circular Economy 2025: 8 Game-Changing Market ... [https://www.researchandmetric.com/blog/circular-economy-2025-insights/]
- 82. Assessment of organizations' maturity towards circular ... [https://www.sciencedirect.com/science/article/pii/S277239092500085X]
- 83. Top 25 Industries in Circular Economy Market (2025-2035) [https://www.sphericalinsights.com/blogs/top-25-industries-in-circular-economy-market-2025-2035-expert-view-by-spherical-insights]
- 84. Resource Utilization of Solid Waste and Circular Economy [https://www.mdpi.com/2076-3417/15/16/9098]
- 85. Sustainable Packaging: Top 20 Innovations for 2025 [https://earth5r.org/sustainable-packaging-top-20-innovations-for-2025/]
- 86. Chemistry needs to be more sustainable. Here's what ... [https://www.soci.org/news/2025/6/chemistry-needs-to-be-more-sustainable]
- 87. New federal report outlines strategic goals to advance ... - C&EN [https://cen.acs.org/acs-news/New-federal-report-outlines-strategic/103/web/2025/02]
- 88. Progress and future outlook towards a safe ... [https://www.nature.com/articles/s42004-025-01785-8]
- 89. Skills shortages threaten circular economy progress [https://www.rsc.org/news/new-report-warns-skills-shortages-threaten-circular-economy-progress]
- 90. Green Chemistry for Climate and Sustainability Certificate ... [https://environment.yale.edu/certificates/greenchemistry]
- 91. Graduate Programs in Green and Sustainable Chemistry [https://chemistryforsustainability.org/learn/graduate-programs-green-and-sustainable-chemistry]
- 92. Chemistry's Pollution Blind Spot: How CMU Researchers ... [https://www.cmu.edu/mcs/news-events/2025/0707-sustainability-story-initiator]
- 93. Leveraging stringency and lifecycle thinking to advance ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11797384/]
- 94. What is ESG Reporting? A comprehensive guide [https://www.sap.com/resources/esg-reporting-guide]
- 95. How Life Cycle Assessment Supports Sustainability ... [https://www.mdpi.com/2673-4591/90/1/56]
- 96. Life Cycle Assessment (LCA) – Everything you need to know [https://ecochain.com/blog/life-cycle-assessment-lca-guide/]
- 97. Adoption of Life Cycle Thinking: Impact-driven comparative ... [https://www.sciencedirect.com/science/article/pii/S0360132324009739]
- 98. 45 ESG indicators explained - The guide to choose yours [https://www.greenscope.io/en/esg/indicators]
- 99. Embracing Sustainable Processes in the Pharmaceutical ... [https://scifiniti.com/3079-1421/1/2025.0013]
- 100. Green Chemistry Challenge Award [https://www.acs.org/funding/awards/green-chemistry-challenge-award.html]
- 101. Sustainable Pharma Labs Using Green Chemistry [https://ispe.org/pharmaceutical-engineering/ispeak/sustainable-pharma-labs-using-green-chemistry]
- 102. What Pharma Needs to Know About Green Chemistry [https://www.news-medical.net/life-sciences/What-Pharma-Needs-to-Know-About-Green-Chemistry.aspx]
- 103. Press release: The Nobel Prize in Chemistry 2005 [https://www.nobelprize.org/prizes/chemistry/2005/press-release/]
- 104. The Nobel Prize in Chemistry 2001 - NobelPrize.org [https://www.nobelprize.org/prizes/chemistry/2001/summary/]
- 105. Green chemistry [https://en.wikipedia.org/wiki/Green_chemistry]
- 106. The Nobel Prize in Chemistry 2001 - Popular information [https://www.nobelprize.org/prizes/chemistry/2001/popular-information/]
- 107. The Nobel Prize in Chemistry 2005 - NobelPrize.org [https://www.nobelprize.org/prizes/chemistry/2005/summary/]
- 108. Trio wins Nobel Prize for 'green chemistry' [https://www.nbcnews.com/id/wbna9595718]
- 109. Green Chemistry Approaches in Pharmaceutical Synthesis [https://www.mdpi.com/2673-9623/5/2/13]
- 110. The twelve goals of circular analytical chemistry [https://www.sciencedirect.com/science/article/abs/pii/S0165993624001687]
- 111. Principles of green chemistry: building a sustainable future [https://link.springer.com/article/10.1007/s44371-025-00152-9]
- 112. January 2025 – Green Chemistry Blog [https://blogs.rsc.org/gc/2025/01/]
- 113. HPLC 2025 Preview: The Road To Sustainable Analytical ... [https://www.chromatographyonline.com/view/hplc-2025-preview-the-road-to-sustainable-analytical-chemistry]
- 114. Introduction to the circular economy themed collection [https://pubs.rsc.org/en/content/articlehtml/2025/su/d5su90007g]
- 115. Call For Papers: Nobel Symposium 2025: The Future of ... [https://axial.acs.org/earth-space-and-environmental-chemistry/call-for-papers-nobel-symposium-2025-the-future-of-chemical-safety-and-sustainable-materials-chemistry]
- 116. Metrics are the key: development of criteria and indicators for ... [https://pubs.rsc.org/en/content/articlehtml/2025/su/d5su00135h]
- 117. Machine learning for catalysis: Bridging data-driven ... [https://www.sciencedirect.com/science/article/abs/pii/S2468519425005415]
- 118. Catalysis meets machine learning: a guide to data-driven ... [https://pubs.rsc.org/en/content/articlehtml/2025/cc/d5cc05274b?page=search]
- 119. Smart Catalysts: How AI is Designing More Efficient ... [https://www.chemcopilot.com/blog/smart-catalysts-how-ai-is-designing-more-efficient-chemical-reactions]
- 120. Interpretable Machine Learning-Guided Plasma Catalysis for ... [https://communities.springernature.com/posts/interpretable-machine-learning-guided-plasma-catalysis-for-hydrogen-production]
- 121. Current progress on bio-based polymers and their future trends [https://pmc.ncbi.nlm.nih.gov/articles/PMC5151099/]
- 122. The Global Market for Bio-based Polymers 2025-2035 [https://www.futuremarketsinc.com/the-global-market-for-bio-based-polymers-2025-2035/]
- 123. Braskem unveils bio-based product innovations at K 2025 [https://www.braskem.com.br/europe/news-detail/braskem-unveils-bio-based-product-innovations-at-k-2025]