Supercritical CO2 Extraction: A Comprehensive Guide for Pharmaceutical Research and Development
This article provides a thorough examination of supercritical CO2 extraction (SFE-CO2) for researchers and drug development professionals.
Supercritical CO2 Extraction: A Comprehensive Guide for Pharmaceutical Research and Development
Abstract
This article provides a thorough examination of supercritical CO2 extraction (SFE-CO2) for researchers and drug development professionals. It covers the fundamental principles of SFE-CO2, explores diverse methodologies and their specific pharmaceutical applications, details strategies for process optimization and troubleshooting, and offers a critical validation against traditional extraction techniques. The content synthesizes current research, including the use of machine learning for solubility prediction and co-solvents for enhanced bioactive compound recovery, to serve as a foundational resource for implementing this green technology in pharmaceutical innovation.
Principles and Foundations of Supercritical CO2 Extraction
A supercritical fluid is a substance maintained above its critical temperature and critical pressure, where it no longer behaves as a typical liquid or gas [1] [2]. In this supercritical state, the substance adopts properties that are intermediate between the two phases, exhibiting gas-like diffusion and viscosity alongside liquid-like density and solvent power [3]. Carbon dioxide (COâ‚‚) is the most prevalent supercritical fluid used in research and industry due to its accessible critical point, low toxicity, and environmental acceptability [4]. Understanding the fundamental thermodynamics of the supercritical state, particularly for COâ‚‚, is crucial for leveraging its unique properties in applications ranging from pharmaceutical extraction to advanced power cycles [1] [5].
This guide details the core principles of the supercritical state of COâ‚‚, with a specific focus on its relevance to extraction research. It provides the quantitative data, theoretical frameworks, and experimental methodologies necessary for scientists to effectively design and optimize processes utilizing supercritical COâ‚‚ (scCOâ‚‚).
The Critical Point of Carbon Dioxide
The critical point of a pure substance is the unique condition of temperature and pressure at which its liquid and gaseous phases coalesce into a single fluid phase [2] [6]. At this point, the meniscus between the liquid and gas vanishes, and properties such as density, refractive index, and enthalpy of vaporization become identical [6]. For carbon dioxide, this critical point is defined by precise values of temperature and pressure, as summarized in Table 1.
Table 1: Critical Point Parameters for Carbon Dioxide
| Parameter | Value | Units |
|---|---|---|
| Critical Temperature (T_c) | 304.128 K / 31.978°C / 87.7604°F [1] | Kelvin / Celsius / Fahrenheit |
| Critical Pressure (P_c) | 7.3773 MPa / 73.773 bar / 72.808 atm [1] | Megapascal / Bar / Atmosphere |
Beyond this critical point, COâ‚‚ exists as a supercritical fluid (scCOâ‚‚). A key phenomenon observed near the critical point is critical opalescence, where the fluid appears cloudy due to large-scale density fluctuations that scatter light [2]. This serves as a visual indicator of the phase transition.
The phase behavior of COâ‚‚ can be visualized through a phase diagram, which maps the states of matter (solid, liquid, gas) and the regions where they coexist as a function of temperature and pressure.
Unique Properties of Supercritical COâ‚‚ as a Solvent
Supercritical COâ‚‚ possesses a combination of physico-chemical properties that make it an exceptional and tunable solvent for research and industrial applications, especially in the extraction of sensitive bioactive compounds [1] [3] [4].
- Tunable Solvation Power: The density of scCOâ‚‚, and consequently its solvating power, is highly sensitive to changes in temperature and pressure near the critical point [3]. This allows researchers to fine-tune the solvent's selectivity, enabling the targeted extraction of specific compounds simply by adjusting system parameters [7] [4].
- Transport Properties: scCOâ‚‚ exhibits transport properties that are favorable for extraction. It has gas-like low viscosity and high diffusivity, which facilitate rapid penetration into porous matrices like plant materials. Simultaneously, it possesses liquid-like density, ensuring sufficient solvent capacity to dissolve target solutes [3].
- Environmental and Safety Profile: COâ‚‚ is non-flammable, chemically inert, and virtually non-toxic [1] [4]. It is also readily available in high purity and is considered a "green" solvent, leaving no harmful residue in extracts [4]. This eliminates the need for complex and costly solvent disposal procedures associated with organic solvents like hexane or acetone [1].
- Low-Temperature Processing: With a critical temperature of just 31°C, scCO₂ is ideal for processing thermolabile compounds found in pharmaceuticals and food products. Extractions can be performed at near-ambient temperatures, preventing thermal degradation of the desired products [1] [4].
A pivotal concept in working with scCO₂ is the Widom line, which represents the pseudo-phase transition where the thermodynamic properties of scCO₂ exhibit extreme values [8]. This region, rather than a single point, guides the operation of scCO₂ systems for optimal properties like heat capacity. Recent molecular dynamics simulations have achieved high accuracy (R² > 97.48%) in fitting Widom line expressions for properties such as density and heat capacity, providing a robust guide for system operation [8].
Experimental Protocol: Observing the Critical Point
This protocol allows for the safe visual demonstration of the critical point of COâ‚‚, a foundational experiment for understanding supercritical fluid behavior [6].
Goal
To demonstrate the phase transition of carbon dioxide from a subcritical (liquid-gas coexistence) state through the critical point to a supercritical state and back again [6].
Theory
In a sealed system at subcritical temperatures, liquid CO₂ and its vapor coexist, separated by a clear meniscus. As the system is heated to the critical temperature (~31°C) under sufficient pressure (>7.38 MPa), the meniscus vanishes due to critical opalescence, forming a single supercritical phase. Upon cooling, the process reverses, and the two phases reappear [2] [6].
Materials (The Researcher's Toolkit)
Table 2: Essential Materials for Critical Point Demonstration
| Item | Function | Critical Safety Note |
|---|---|---|
| Sealed Glass Rod containing subcritical liquid COâ‚‚ | High-pressure vessel for observing the phase transition. The liquid COâ‚‚ is in the lower part, saturated vapour above it [6]. | WARNING: The rod is under extreme pressure (>7 MPa). Handle with extreme caution to avoid shocks or drops [6]. |
| Temperature-Controlled Water Bath (capable of 45-50°C) | Provides uniform heating to bring the CO₂ to its critical temperature [6]. | Ensure stable setup to prevent the rod from slipping. |
| Light Source (e.g., Slide Projector) | Backlighting to project a clear image of the meniscus and critical opalescence onto a screen [6]. | - |
| cis-9-Octadecene-1-thiol | cis-9-Octadecene-1-thiol, CAS:31494-22-1, MF:C18H36S, MW:284.5 g/mol | Chemical Reagent |
| Gibbs Reagent | Gibbs Reagent, CAS:101-38-2, MF:C6H2Cl3NO, MW:210.4 g/mol | Chemical Reagent |
Step-by-Step Procedure
- Setup: Fill the beaker with water heated to 45-50°C. Turn on the slide projector and position the CO₂ rod in front of its lamp so the liquid level is projected onto a screen [6].
- Heating (Transition to Supercritical): Immerse the lower end of the rod into the water bath. Warm the rod gradually while observing the projected image. The meniscus will become wavy and eventually vanish, replaced by a cloudy, opalescent appearance that fills the rod as it reaches the critical point [6].
- Cooling (Transition to Subcritical): Remove the rod from the water bath and allow it to cool in air. Observe the reversal of the process: the opalescence clears, and a faint meniscus reforms, separating the liquid and gaseous COâ‚‚ [6].
Sample Results and Interpretation
Successful execution will show the distinct disappearance and reappearance of the liquid-gas boundary. The critical opalescence observed during the transition is caused by the scattering of light from large density fluctuations within the fluid at the critical point [2] [6]. The following workflow summarizes the experimental and application principles of scCOâ‚‚.
Advanced Thermodynamic Properties and Widom Line Region
For advanced research and precise process design, understanding the thermodynamic properties of scCOâ‚‚ beyond the critical point is essential. The Widom line is a key concept, representing an extension of the coexistence line into the supercritical region, where certain thermodynamic response functions reach their maximum [8]. This region, rather than a single line, guides the operation of scCOâ‚‚ systems for optimal properties.
Recent advances in large-scale molecular dynamics (MD) simulations have enabled highly accurate calculation of these properties. A 2025 study calculated key properties with high precision, as shown in Table 3, providing a valuable dataset for researchers [8].
Table 3: Thermodynamic Properties of scCO₂ from Molecular Dynamics Simulation (300–900 K, 7.3773–20 MPa)
| Thermodynamic Property | Average Relative Error (vs. Expected) | Key Application Implication |
|---|---|---|
| Density | 3.76% [8] | Directly determines solvent power and selectivity for extraction. |
| Isobaric Heat Capacity (Cp) | 3.93% [8] | Critical for heat exchanger and reactor design in power cycles. |
| Isochoric Heat Capacity (Cv) | 3.11% [8] | Informs fundamental thermodynamic models. |
| Volume Expansion Coefficient | 5.76% [8] | Affects flow dynamics and buoyancy-driven convection. |
| Isothermal Compression Coefficient | 7.07% [8] | Important for understanding compressibility and pressure propagation. |
The expressions for the Widom lines of these properties were fitted with a coefficient of determination (R²) above 97.48%, creating a reliable map of the "Widom line region" where scCO₂'s properties transition from liquid-like to gas-like [8]. This knowledge allows scientists to precisely control processes by targeting specific pressure-temperature conditions relative to this region.
The unique properties of scCOâ‚‚ are directly harnessed in extraction processes, forming the basis of its utility in pharmaceuticals, food, and cosmetics [7]. Its tunable solvation power allows for selective extraction; for instance, lower pressures can extract essential oils, while higher pressures can extract heavier compounds like waxes [1]. The gas-like transport properties enable deep penetration into botanical matrices, leading to high extraction yields, while the low operating temperature preserves the integrity of thermolabile active pharmaceutical ingredients (APIs) [1] [4]. Finally, the benign environmental profile ensures that the final extract is free of toxic solvent residues, a critical requirement for drug development [1] [4].
In conclusion, a rigorous understanding of the critical point and the unique, tunable solvent properties of supercritical COâ‚‚ is fundamental for researchers. From observing the foundational phase transition in a laboratory rod to applying advanced molecular dynamics simulations for process optimization, this knowledge empowers the development of more efficient, selective, and sustainable extraction technologies in scientific and industrial contexts.
Why CO2? Advantages as a Non-Toxic, Non-Flammable, and Recyclable Solvent
Supercritical carbon dioxide (scCO₂) is carbon dioxide held at or above its critical temperature of 30.98 °C (304.13 K) and critical pressure of 7.3773 MPa (73.8 bar) [1]. In this supercritical state, CO₂ adopts properties midway between a gas and a liquid, expanding to fill its container like a gas but with a density comparable to that of a liquid [1]. This unique combination of properties makes it an exceptionally effective and versatile solvent for extraction, separation, and purification processes across pharmaceutical, food, and cosmetic industries.
The role of scCOâ‚‚ within broader supercritical COâ‚‚ extraction research is pivotal, as it represents a paradigm shift toward sustainable and green chemistry principles in industrial solvent applications. Its tunable physical properties, coupled with an exceptional safety and environmental profile, offer a viable alternative to conventional organic solvents, which are increasingly scrutinized for their toxicity, flammability, and environmental impact [9] [10]. This technical guide details the fundamental advantages of COâ‚‚, supported by quantitative data, experimental methodologies, and visualizations tailored for researchers and drug development professionals.
Fundamental Advantages of COâ‚‚ as a Solvent
Non-Toxic and Safe Profile
Carbon dioxide is generally recognized as safe (GRAS) by regulatory authorities such as the U.S. Food and Drug Administration (FDA) [11] [12]. This classification is crucial for applications in the food, pharmaceutical, and nutraceutical industries, where solvent residues in final products are a major concern.
- Non-Toxic and Non-Flammable: Unlike traditional solvents such as hexane, methanol, or acetone, COâ‚‚ poses no inherent toxicity or flammability risks [9] [13] [12]. This eliminates significant safety hazards associated with solvent handling, storage, and industrial processing, reducing the need for specialized hazardous location-rated environments [12].
- Solvent-Residue-Free Extracts: The supercritical COâ‚‚ extraction process leaves no solvent residues in the final extract [11] [13] [14]. This is achieved through simple depressurization, which reverts supercritical COâ‚‚ to a gaseous state, leaving behind a pure extract without the need for energy-intensive secondary purification steps [11]. This ensures the safety and purity of extracts intended for consumer products [11].
Environmental and Recyclability Benefits
The environmental benefits of using COâ‚‚ as a solvent are multifaceted, impacting both direct process efficiency and broader ecological footprints.
- Recyclable and Abundant: The COâ‚‚ used in industrial processes is typically captured as a byproduct from other large-scale industrial processes, such as fertilizer production or from geothermal sources [11] [12]. This makes it abundant, readily available, and contributes to a reduced carbon footprint [11] [14]. Furthermore, in a closed-loop supercritical extraction system, COâ‚‚ can be continuously recycled and reused after the extraction and separation steps, significantly reducing solvent consumption and waste [10] [13].
- Reduced Environmental Impact: scCOâ‚‚ extraction replaces petroleum-based and halogenated organic solvents, which are complex to manage, require expensive disposal procedures, and can contribute to environmental pollution [11] [10]. The process is considered carbon-neutral as it typically uses captured COâ‚‚ and does not generate new COâ‚‚ emissions [14]. Additionally, the spent biomass from scCOâ‚‚ extraction is typically non-hazardous, simplifying waste disposal [14].
Tunable Solvent Properties
One of the most powerful features of supercritical COâ‚‚ is its tunable solvent power [9] [12]. The density of scCOâ‚‚, and consequently its solvating power, can be precisely and continuously modulated through incremental changes in pressure and temperature [10].
- Selective Extraction: This tunability allows researchers to fine-tune the process for superior selectivity [9] [13]. It enables the selective isolation of target compounds by adjusting operational parameters to solubilize specific components while leaving others behind [1]. This can be leveraged for fractional separation, where different compounds in a mixture are sequentially separated by manipulating pressure and temperature in a series of separators [15].
- Preservation of Heat-Sensitive Compounds: The relatively low critical temperature of CO₂ (31.1°C) permits extractions to be conducted under mild thermal conditions [9] [16]. This is critical for processing thermally labile bioactive compounds, such as many pharmaceuticals and essential oils, preventing their degradation and preserving their biological activity [9] [10].
Table 1: Critical Point Comparison of Common Supercritical Fluids
| Solvent | Critical Temperature (°C) | Critical Pressure (bar) | Critical Density (kg/m³) |
|---|---|---|---|
| Carbon Dioxide (COâ‚‚) | 31.1 [1] [10] | 73.8 [1] [13] | 467.6 [10] |
| Water (Hâ‚‚O) | ~374 [10] | ~221 [10] | 322 |
| Ethane (C₂H₆) | 32.2 | 48.8 | 203 |
| Nitrous Oxide (Nâ‚‚O) | 36.4 | 72.4 | 457 |
Table 2: Comparison of Physical Properties of Gases, Supercritical COâ‚‚, and Liquids
| Physical Property | Gas | Supercritical COâ‚‚ | Liquid |
|---|---|---|---|
| Density (kg/m³) | 0.6-2.0 [10] | 200-900 [10] (Tunable) | 600-1600 [10] |
| Viscosity (Pa·s) | 0.01-0.03 [10] | 0.01-0.09 [10] | 0.2-3.0 [10] |
| Diffusivity (cm²/s) | 0.01-0.04 [10] | 0.07-0.2 [10] | < 0.00005 [10] |
Experimental Protocols and Methodologies
Standard Supercritical COâ‚‚ Extraction Workflow
A typical laboratory-scale supercritical COâ‚‚ extraction process follows a systematic protocol to ensure reproducibility and efficiency. The following methodology is adapted for the extraction of bioactive compounds from plant materials (e.g., herbs, leaves) [13] [14].
Principle: To utilize the tunable solvating power of supercritical COâ‚‚ to extract target compounds from a solid matrix, followed by separation via depressurization.
Materials and Reagents:
- Raw Material: Dried and finely ground plant matter (e.g., Artemisia annua leaves).
- Extraction Solvent: High-purity carbon dioxide (COâ‚‚) gas supply.
- Optional Co-solvent: Food-grade or HPLC-grade ethanol or methanol.
- Equipment: Supercritical fluid extraction system comprising a COâ‚‚ pump, co-solvent pump (if used), pre-heater, extraction vessel, pressure control valves (e.g., back-pressure regulators), one or more separators, and a COâ‚‚ recycling or venting system.
Procedure:
- Sample Preparation: The raw plant material is dried to a low moisture content (increased moisture reduces extraction efficiency [16]) and milled to a consistent particle size (e.g., 0.2-0.5 mm) to increase the surface area for mass transfer [16].
- System Pressurization and Heating:
- The prepared sample is loaded into the extraction vessel.
- The system is sealed and brought to the desired operational temperature using the pre-heater and vessel heating jacket.
- COâ‚‚ is pumped into the system until the target pressure is achieved, bringing the COâ‚‚ to its supercritical state.
- If a co-solvent is used (e.g., 1-10% ethanol), it is introduced via a separate pump and mixed with the scCOâ‚‚ stream before entering the extraction vessel [9].
- Static and Dynamic Extraction:
- The system may be held under static conditions (no flow) for a predetermined time (e.g., 15-30 minutes) to allow for saturation.
- The supercritical COâ‚‚ is then allowed to flow dynamically through the vessel at a controlled flow rate (e.g., 1-10 g/min) for a set duration (e.g., 1-4 hours), dissolving the target compounds.
- Separation and Collection:
- The COâ‚‚-rich stream containing the dissolved solutes passes from the extractor into a separator.
- In the separator, the pressure is precisely reduced using a back-pressure regulator, causing a drastic drop in the solvent power of COâ‚‚ [14] [15].
- The extracted compounds precipitate out and are collected from the bottom of the separator.
- For complex mixtures, multiple separators in series can be used, each at successively lower pressures, to fractionate different classes of compounds based on their solubility [15].
- Solvent Recovery: The now-gaseous COâ‚‚ exits the separator. It can be vented or, in a closed-loop system, recompressed and recycled back to the COâ‚‚ pump, significantly reducing solvent consumption [11] [10].
Protocol for Fractional Separation of Extract Components
Fractional separation, or fractionation, is a powerful technique to isolate specific components from a complex extract [15].
Principle: To separate a mixture into its individual components by leveraging their different dissolution pressures in scCOâ‚‚ through a multi-stage separation process.
Procedure:
- Primary Extraction: Perform steps 1-3 from the standard extraction protocol above. The output is a scCOâ‚‚ stream containing a mixture of dissolved compounds.
- Multi-Stage Separation:
- The mixture is passed through a series of separators (e.g., Separator 1, Separator 2).
- Separator 1 is maintained at a specific high pressure (e.g., 150 bar) and temperature. At these conditions, only the least soluble component(s) precipitate, while others remain dissolved in the scCOâ‚‚.
- The stream then flows to Separator 2, which is maintained at a lower pressure (e.g., 80 bar) and temperature. This second pressure drop causes the next fraction of compounds to precipitate.
- Precise pressure control in each separator, achieved using specialized back-pressure regulators, is critical for obtaining pure fractions [15].
- Collection: Each separator yields a distinct fraction of the extract. The final gaseous COâ‚‚ is recycled.
Diagram 1: SCO2 Extraction and Fractionation Workflow.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of supercritical COâ‚‚ extraction requires specific reagents and equipment. The following table details key components of a research-scale setup.
Table 3: Essential Materials and Reagents for Supercritical COâ‚‚ Extraction Research
| Item | Function/Description | Research Considerations |
|---|---|---|
| High-Purity COâ‚‚ Supply | Primary extraction solvent. Must be free of impurities that could contaminate the extract or interfere with the process. | Purity > 99.9% is typical. Source (bulk tank or cylinders) impacts long-term operational costs [12]. |
| Co-Solvents | Modifies the polarity of scCOâ‚‚ to enhance solubility of target compounds. | Ethanol is common due to its GRAS status [9]. Methanol offers higher polarity. Concentration (1-15%) is optimized empirically [9]. |
| Extraction Vessel | High-pressure cell that holds the solid sample during extraction. | Constructed from 316 stainless steel. Volume determines batch size. Must withstand pressures up to 700 bar [12]. |
| Precision Pumps | To deliver COâ‚‚ (and co-solvent) at a constant, precise flow rate against high back-pressure. | Syringe pumps offer high precision for lab-scale work. Dual-pump systems allow independent control of COâ‚‚ and co-solvent flows [17]. |
| Back-Pressure Regulator (BPR) | Maintains consistent system pressure upstream. Critical for fractionation and controlling solvent power. | Must provide precise pressure control and resist blockage from ice formation during COâ‚‚ expansion [15]. Diaphragm-based regulators are often used. |
| Heated Oven/Jacket | Maintains the entire system (vessels, lines) at a temperature above the critical point of CO₂. | Requires precise temperature control (±1°C) to ensure stable supercritical conditions and reproducible results [13]. |
| Separator(s) | Vessel(s) where pressure is reduced to precipitate the extract. | Multiple separators enable fractionation. Often equipped with sight glasses and temperature control [14] [15]. |
| Senkirkin | Senkirkin, CAS:2318-18-5, MF:C19H27NO6, MW:365.4 g/mol | Chemical Reagent |
| Thozalinone | Thozalinone, CAS:655-05-0, MF:C11H12N2O2, MW:204.22 g/mol | Chemical Reagent |
Quantitative Data and Solubility Behavior
The efficiency of scCOâ‚‚ extraction is governed by the solubility of target compounds, which is a direct function of pressure and temperature. The following data illustrates key relationships.
Table 4: Influence of Operational Parameters on Extraction Yield and Selectivity
| Parameter | Effect on Process | Typical Experimental Range | Impact on Yield/Selectivity |
|---|---|---|---|
| Pressure | Directly controls COâ‚‚ density and solvent power. | 80 - 600 bar [16] [10] | Increased pressure generally increases yield for most compounds (e.g., lipids, essential oils) by enhancing solubility [16] [14]. |
| Temperature | Has a dual effect: increases solute vapor pressure but decreases CO₂ density. | 35 - 80 °C [10] | The net effect is compound-specific. A "crossover region" exists where solubility is influenced by the competing effects [10]. |
| COâ‚‚ Flow Rate | Influences the mass transfer kinetics and extraction time. | 1 - 10 g/min (lab-scale) | Higher flow reduces extraction time but may decrease efficiency if equilibrium is not reached. Optimized to balance throughput and COâ‚‚ consumption [17]. |
| Particle Size | Affects the diffusion path length and internal mass transfer resistance. | 0.1 - 0.5 mm [16] | Smaller particles increase yield and rate by increasing surface area, but excessive fining can cause channeling [16]. |
Diagram 2: Primary Pressure Effect on Extraction Yield.
The solubility of a model compound like naphthalene in scCO₂ vividly demonstrates this pressure dependence. At a constant temperature of 50°C, the solubility can increase from a negligible 0.1 wt% at 70 atm to a significant 10 wt% at 300 atm [14]. This strong dependence is the foundational principle for both extraction and fractional separation.
Supercritical carbon dioxide stands as a superior solvent choice for modern, sustainable research and industrial processes due to its compelling combination of safety, environmental, and tunable physicochemical properties. Its status as a non-toxic, non-flammable, and recyclable solvent aligns perfectly with the principles of green chemistry, while its tunability offers researchers unparalleled control over separation processes. The experimental protocols and data outlined in this guide provide a foundation for scientists and drug development professionals to harness this versatile technology, contributing to safer, cleaner, and more efficient extraction methodologies in line with the evolving demands of scientific discovery and regulatory standards.
Core Components of a Supercritical CO2 Extraction System
Supercritical Carbon Dioxide (SC-CO2) Extraction is a sophisticated separation technology that utilizes carbon dioxide above its critical temperature (31.1 °C) and pressure (73.9 bar) as a solvent [18] [19]. In this supercritical state, CO2 exhibits unique properties, combining the penetrative ability of a gas with the solvating power of a liquid, making it an exceptionally efficient extraction medium [14]. This guide details the core components of an SFE system, providing researchers and drug development professionals with the technical foundation necessary for implementing this technology in analytical and process-scale applications across natural product extraction, pharmaceutical compound isolation, and nutraceutical development [20].
Core Components and Their Functions
A supercritical CO2 extraction system is an integrated assembly of several specialized components designed to maintain CO2 in its supercritical state throughout the extraction process. The synergy between these components ensures efficient, reproducible, and safe operation [18] [19].
Table 1: Core Components of a Supercritical CO2 Extraction System
| Component | Primary Function | Technical Specifications & Common Types |
|---|---|---|
| CO2 Supply & Pumping System | Pressurizes liquid CO2 to supercritical conditions [18]. | Pump Types: Reciprocating or syringe pumps (small scale); Diaphragm pumps (large scale) [18].Requirement: CO2 is typically cooled (e.g., below 5°C) before pumping to maintain liquid state and ensure pump efficiency [18]. |
| Extraction Vessel | Holds the solid or liquid raw material (matrix) for extraction [18] [19]. | Pressure Rating: Must withstand high pressures, typically at least 74 bar and often up to 350-800 bar [18].Heating: Jacketed or placed in an oven to maintain temperature above critical point [18]. |
| Heating System / Oven | Heats the pressurized CO2 and extraction vessel to achieve and maintain supercritical conditions [18] [20]. | Precisely controls extraction temperature, which significantly impacts solvent density and selectivity [18] [21]. |
| Pressure Maintenance Device | Maintains system pressure upstream. | Types: Capillary restrictor (analytical scale), needle valve, or automated Back Pressure Regulator (BPR) [18] [15]. Challenge: Requires heating to prevent freezing from adiabatic CO2 expansion [18] [15]. |
| Separation Vessel (Separator) | Receives CO2-extract mixture; precipitates extract by reducing CO2 solvating power [18] [19]. | Method: Pressure reduction, temperature increase, or both [18] [14]. Multiple separators in series enable fractional separation of different compounds [15]. |
| Heat Exchangers | Cools CO2 pre-pump and heats/cools streams at various process points [18] [20]. | Manages thermal energy to maintain specific conditions and counteract cooling from CO2 expansion [18]. |
| CO2 Recycling System | Cools, re-liquefies, and recirculates CO2 gas from the separator, reducing operational costs and environmental impact [19] [14]. | Essential for large-scale industrial processes to ensure economic viability [14]. |
The following diagram illustrates the logical workflow and the interconnection of these core components in a typical supercritical CO2 extraction system.
Advanced System Configuration: Fractional Separation
For complex extracts, a single separator is often insufficient. Fractional Separation employs multiple separators in series, each set at a progressively lower pressure (and/or different temperature), to selectively precipitate different classes of compounds based on their varying solubility in CO2 [15]. This multi-stage process, controlled by precise back-pressure regulators, enables the selective and sequential isolation of specific components from a complex mixture, greatly enhancing the selectivity and purity of the final products [15] [20].
Detailed Experimental Protocol for System Operation
To ensure reproducible results, a standardized operational sequence must be followed. The protocol below synthesizes general best practices with specific parameters from published research on extracting lycopene from grapefruit [21] and bioactive compounds from jamun fruit [22].
Raw Material Preparation
- Commutation: The plant material (e.g., grapefruit, jamun pulp) must be dried and ground into a fine powder [21] [22]. A particle size of 250 µm (passed through a 40-60 mesh sieve) is often optimal to increase surface area for extraction while avoiding excessive packing that can impede fluid flow [21].
- Loading: The prepared biomass is mixed with an inert material like glass wool and loaded into the extraction vessel to prevent channeling and ensure uniform solvent passage [22]. For the referenced study, a 100 g sample was used [21].
System Pressurization and Heating
- The system is sealed, and liquid CO2 is pumped into the extraction vessel.
- Pressure and Temperature: The system is brought to the desired operating conditions. For example, a study might use 305 bar and 70 °C [21]. The pressure and temperature are maintained by the pump and heating system, respectively.
Dynamic Extraction
- Supercritical CO2 is continuously passed through the extraction vessel at a controlled mass flow rate. A common flow rate in research settings is 35 g/min [21].
- Use of Co-solvent: To enhance the extraction of polar compounds, a food-grade co-solvent like ethanol can be introduced. It is typically added at 2-5% of the total solvent volume [21] [22]. A co-solvent pump is used for this purpose.
Separation and Collection
- The CO2-rich stream containing the dissolved solutes passes through a back-pressure regulator into the separation vessel.
- In the separator, a reduction in pressure (e.g., to atmospheric or a controlled intermediate pressure) causes the CO2 to revert to a gaseous state, precipitating the extracted material [18] [19]. The extract is collected from the separator valve.
Solvent Recycling and System Depressurization
- The gaseous CO2 exiting the separator can be vented or directed to a recycling system where it is cooled, re-liquefied, and returned to the CO2 supply tank for reuse [14].
- After the set extraction time (e.g., 135 minutes [21]), the pump is stopped, and the system is slowly depressurized.
Table 2: Example Experimental Parameters from Peer-Reviewed Studies
| Extraction Target | Optimal Pressure | Optimal Temperature | CO2 Flow Rate | Co-solvent (Ethanol) | Extraction Time | Reference |
|---|---|---|---|---|---|---|
| Lycopene from Grapefruit | 305 bar | 70 °C | 35 g/min | 5% | 135 min | [21] |
| Bioactives from Jamun Fruit | 162 bar | 50 °C | Not Specified | 2.0 g/min (flow) | Not Specified | [22] |
The Scientist's Toolkit: Essential Research Reagents & Materials
Successful supercritical CO2 extraction relies on more than just equipment. The following table lists key consumables and materials essential for research and development in this field.
Table 3: Essential Research Reagents and Materials for SFE
| Reagent/Material | Function in SFE Research | Technical Notes |
|---|---|---|
| SFE-Grade CO2 | Primary extraction solvent. | High purity (≥ 99.9%) is essential to prevent contamination and ensure consistent solvent strength [22]. |
| Food-Grade Co-solvents (e.g., Ethanol) | Modifies polarity of SC-CO2 to enhance extraction efficiency of polar compounds (e.g., polyphenols, anthocyanins) [18] [22]. | Preferred for food and pharmaceutical applications. Must be anhydrous to prevent ice formation during expansion [21] [20]. |
| Raw Biomass | The source material for extraction. | Requires standardized pre-processing (drying, milling, sieving) for reproducible results [21] [22]. |
| Analytical Standards | Used for quantifying and identifying extracted compounds via techniques like HPLC, GC-MS, or SFC [21] [22]. | Critical for method validation and accurate yield calculation (e.g., using cyanidin-3-glucoside for anthocyanins [22]). |
| Inert Packing Material | Used to fill void space in the extraction vessel, improving flow dynamics and preventing channeling [22]. | Glass wool is a common example used to ensure uniform packing and solvent distribution [22]. |
| Orforglipron | Orforglipron, CAS:2212020-52-3, MF:C48H48F2N10O5, MW:883.0 g/mol | Chemical Reagent |
| 3-(N-methyl4-methylbenzenesulfonamido)-N-{[3-(trifluoromethyl)phenyl]methyl}thiophene-2-carboxamide | 3-(N-methyl4-methylbenzenesulfonamido)-N-{[3-(trifluoromethyl)phenyl]methyl}thiophene-2-carboxamide, CAS:1115871-56-1, MF:C21H19F3N2O3S2, MW:468.51 | Chemical Reagent |
The supercritical CO2 extraction system is a sophisticated integration of components designed to precisely control the physical state and solvating power of carbon dioxide. Understanding the role of each core part—from the high-pressure pump and extraction vessel to the separator and recycling system—is fundamental for researchers aiming to develop efficient, scalable, and reproducible extraction protocols. The adaptability of the system, particularly through the use of co-solvents and fractional separation, allows for remarkable selectivity, making it an indispensable tool in modern natural product and pharmaceutical research. By adhering to detailed experimental protocols and utilizing high-quality reagents, scientists can leverage this green technology to isolate high-purity bioactive compounds effectively.
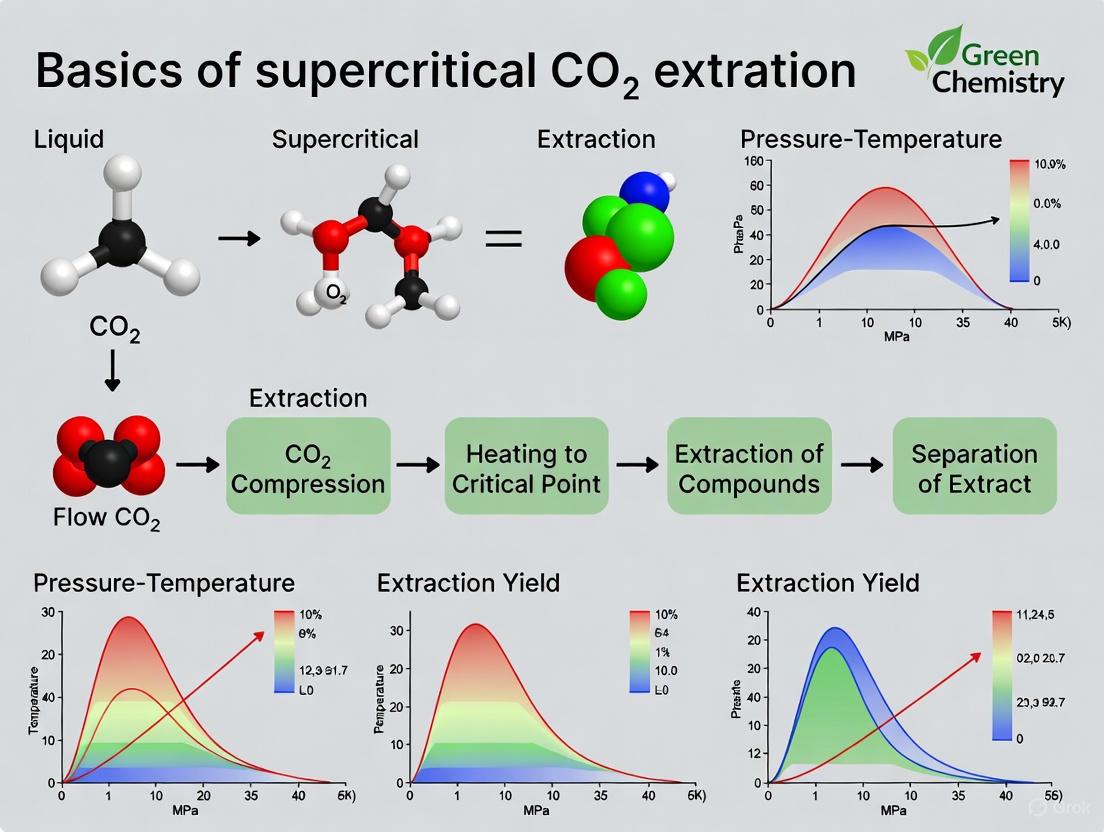
Supercritical fluid extraction using carbon dioxide (SFE-CO2) is a advanced separation process that utilizes carbon dioxide above its critical temperature and pressure as the primary solvent to isolate one component (the extractant) from another (the matrix) [18]. This technology has gained significant prominence as a green and sustainable method, particularly in pharmaceutical, food, and cosmetic industries, due to its ability to eliminate the need for hazardous organic solvents while protecting heat-sensitive bioactive compounds [9]. The core principle leverages the unique properties of supercritical CO2, which exhibits liquid-like densities with gas-like diffusivity and viscosity, enabling superior penetration into solid matrices and efficient extraction [18] [16].
The critical point of carbon dioxide is defined by a temperature of 31°C and a pressure of 74 bar [18]. Beyond this point, CO2 enters a supercritical state that is neither liquid nor gas but possesses properties of both, making it an exceptionally tunable solvent. The tunable dissolving power of supercritical CO2, achieved by simply varying pressure and temperature conditions, allows for remarkable selectivity in extracting target compounds without thermal degradation [18] [23]. This technical guide provides a comprehensive breakdown of the fundamental SFE-CO2 process, detailing each operational stage from initial pressurization to final collection, and situates this technology within broader research on sustainable extraction methodologies.
The SFE-CO2 System: Core Components and Functions
A typical SFE-CO2 system consists of several integrated components that work in concert to maintain supercritical conditions and achieve efficient extraction. Each component plays a critical role in the process integrity and efficiency.
CO2 Supply and Pump: Liquid carbon dioxide is supplied from a reservoir or cylinder and pumped as a liquid, typically below 5°C and approximately 50 bar pressure [18]. Pumping CO2 as a liquid is essential for efficiency, as liquids are nearly incompressible; pumping supercritical fluid would consume much of the pump stroke in compression rather than fluid transfer [18]. For small-scale extractions (up to a few grams per minute), reciprocating CO2 pumps or syringe pumps are common, while diaphragm pumps are preferred for larger scale operations [18].
Pressure Vessel (Extraction Cell): This vessel contains the sample matrix and must withstand high pressures—typically at least 74 bar, with most extractions conducted below 350 bar, though some applications (e.g., vegetable oil extraction) may require pressures up to 800 bar [18]. The vessel requires precise temperature control, achieved through placement in an oven for small systems or via heated jackets for larger vessels [18].
Pressure Maintenance System: The system pressure must be maintained consistently from the pump through the pressure vessel. In smaller systems, this is often accomplished with a simple restrictor (capillary tube or needle valve), while larger systems employ back pressure regulators that maintain upstream pressure via spring, compressed air, or electronically controlled valves [18]. A critical consideration is the heating of this component, as adiabatic expansion of CO2 causes significant cooling that can lead to blockages from frozen water or extracted materials [18].
Collection Vessel: This is where the extracted material is ultimately recovered. The supercritical solvent, now laden with solubilized compounds, passes into this vessel at lower pressure, causing a sharp decrease in CO2 density and dissolving power, which precipitates the extracted material [18]. For analytical-scale SFE, the gaseous CO2 is often bubbled through a solvent trap after depressurization to capture the precipitated components [18].
Heating and Cooling System: Temperature management is crucial throughout the system. The CO2 must be cooled before pumping to maintain liquid conditions, heated after pressurization to achieve supercriticality, and the separator may require heating to prevent cooling during expansion [18].
Step-by-Step Process Breakdown
The SFE-CO2 process follows a logical sequence where each step directly influences extraction efficiency and selectivity.
Step 1: Preparation of the Matrix
The solid matrix containing the target compounds must undergo specific preparation steps to maximize extraction efficiency. The sample is typically freeze-dried and ground to a fine powder with a particle size ranging from 0.4–0.8 mm [24]. Reducing particle size increases the surface area for mass transfer, while freeze-drying removes moisture that can interfere with extraction efficiency [24]. The prepared sample is then precisely loaded into the extraction vessel.
Step 2: Pressurization and Heating
Liquid CO2 is pumped from its reservoir into the system. The pump must deliver CO2 at a pressure exceeding the critical pressure of 74 bar [18]. The mass flow rate should be carefully controlled and measured using Coriolis flow meters, as the density of CO2 changes with temperature [18]. The pressurized liquid CO2 then passes through a heating zone where it is heated above its critical temperature of 31°C, transitioning it into a supercritical state before it enters the extraction vessel [18].
Step 3: Supercritical Extraction
Once in the extraction vessel, the supercritical CO2 rapidly diffuses into the solid matrix, dissolving the target material [18]. The process involves two essential mass transfer steps: (1) diffusion of the solvent into the matrix, and (2) dissolution of the material into the supercritical fluid followed by its diffusion out of the matrix into the bulk solvent [18]. The properties of the supercritical fluid can be altered by varying pressure and temperature, allowing for selective extraction—for instance, volatile oils can be extracted at lower pressures (around 100 bar), while lipids require higher pressures [18]. The duration of this dynamic extraction phase varies significantly based on the application, ranging from 10-60 minutes for some materials to several hours for others [18] [24].
Step 4: Depressurization and Separation
The solution of dissolved extract in supercritical CO2 is swept from the extraction cell into a separator or collection vessel maintained at lower pressure [18]. This pressure reduction dramatically decreases the density and solvating power of the CO2, causing the extracted material to precipitate out [18]. It is possible to implement fractionation using a series of vessels at progressively reducing pressures to separate different compound classes based on their solubility characteristics [18].
Step 5: Collection and Solvent Recovery
The precipitated extract is collected in the separation vessel. Meanwhile, the CO2 can follow one of two paths: (1) it can be cooled, re-compressed, and recycled back to the pump to minimize solvent consumption and operational costs, or (2) it can be depressurized to atmospheric pressure and vented [18] [25]. For analytical applications where solvent recycling isn't implemented, the gaseous CO2 is typically bubbled through a solvent to trap any remaining precipitated components [18].
Step 6: System Cleanup
Following collection, the system must be purged of any residual extract to prevent cross-contamination between batches. The extraction vessel requires cleaning, and all components should be inspected for potential clogging or wear, particularly when processing materials with high lipid or moisture content [18].
The following workflow diagram illustrates the complete SFE-CO2 process:
Process Modeling and Mass Transfer Fundamentals
The efficiency of SFE-CO2 is governed by fundamental mass transfer principles. A simple model conceptualizes two essential steps: (1) transport of the solid particles to the surface (via diffusion or other mechanisms), and (2) dissolution in the supercritical fluid [18]. The relative rates of these steps determine the overall extraction kinetics and can be visualized through concentration profiles within a spherical particle [18].
In scenarios where dissolution is fast relative to diffusion, material is carried away from the particle edge faster than it can diffuse from the center, resulting in a concentration profile that drops to zero at the surface [18]. This creates a diffusion-limited extraction where the rate can be increased by raising temperature to enhance diffusion, but not by increasing solvent flow rate [18]. Conversely, when solubility is low relative to diffusion, the extractant diffuses to the edge faster than the solvent can carry it away, resulting in a relatively flat concentration profile across the particle [18]. This solubility-limited extraction can be accelerated by increasing solvent flow rate [18].
The extraction curve (% recovery vs. time) reveals the dominant mechanism: diffusion-controlled extractions show initially rapid rates that slow dramatically once surface concentration drops to zero; solubility-limited extractions demonstrate nearly constant rates until completion; and extractions with significant matrix effects (e.g., desorption from active sites) may plateau before achieving complete recovery [18].
Optimization of SFE-CO2 Parameters
Optimizing SFE-CO2 requires balancing competing factors of completeness, speed, selectivity, and cost. The optimal configuration depends on the extraction purpose—analytical applications prioritize complete extraction in the shortest time, while production-scale operations may target 70-80% yield for economic reasons, considering solvent consumption and throughput [18].
Key Operational Parameters
- Pressure and Temperature: These interdependent parameters directly control solvent density and selectivity. Generally, higher pressure increases solubility, while temperature effects are more complex—near the critical point, increasing temperature decreases density and dissolving power, but at higher pressures, solubility typically increases with temperature [18].
- CO2 Flow Rate: Measured as mass flow rather than volume due to CO2 compressibility [18]. High flow rates maximize extraction speed but waste solvent in diffusion-limited regimes, while low flows minimize solvent use but extend processing time significantly [18].
- Extraction Time: Must be optimized for each application. Research indicates that dynamic extraction times can range from 40-70 minutes for initial optimization [24] to 7 hours for complete extraction of certain compounds like polyprenol [24].
- Co-solvents: Modifiers such as ethanol or methanol can significantly enhance the extraction of polar compounds that have limited solubility in pure CO2 [18] [9]. Food-grade ethanol is particularly valuable in pharmaceutical and food applications, acting as a polarity modifier that expands the range of extractable compounds while maintaining regulatory compliance [18] [9]. Typical co-solvent flow rates are much lower than main CO2 flow, for instance 0.05 mL/min against a CO2 flow of 10 mL/min [24].
Quantitative Process Parameters
The following table summarizes key operational parameters and their effects on SFE-CO2 efficiency, compiled from research applications:
Table 1: SFE-CO2 Operational Parameters and Optimization Guidelines
| Parameter | Typical Range | Effect on Process | Research Example |
|---|---|---|---|
| Pressure | 100-350 bar (up to 800 bar for oils) | Higher pressure increases solvent density and dissolving power; enables selective extraction of different compound classes [18]. | Polyprenol extraction optimized at 200 bar; 300 bar preferred for faster kinetics [24]. |
| Temperature | 40-70°C | Complex effect: near critical point, higher T decreases density; at higher pressures, increases solubility [18] [24]. | 70°C effective for polyprenol extraction from conifers [24]. |
| CO2 Flow Rate | Varies by scale (e.g., 10 mL/min) | Higher flows reduce extraction time but increase solvent use; optimal flow balances time and cost [18] [24]. | Mass flow measurement recommended via Coriolis flow meters [18]. |
| Extraction Time | 10-60 min (up to 7 hours) | Duration depends on matrix and compound; longer times increase completeness but diminish throughput [18] [24]. | 7-hour dynamic extraction provided highest polyprenol yield [24]. |
| Co-solvent (Ethanol) | 0.5-10% modifier | Enhances polarity and solubility of polar compounds; ethanol preferred for food/pharma applications [24] [9]. | 0.05 mL/min ethanol flow rate significantly improved polyprenol recovery [24]. |
| Particle Size | 0.4-0.8 mm | Smaller particles increase surface area and reduce diffusion path length, improving kinetics [24]. | Freeze-dried, homogenized tissue improves extraction efficiency [24]. |
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of SFE-CO2 requires specific materials and reagents tailored to research objectives. The following table details essential components for establishing a robust SFE-CO2 process.
Table 2: Essential Research Reagents and Materials for SFE-CO2
| Item | Specification/Function | Research Application Notes |
|---|---|---|
| Carbon Dioxide | >99.9% purity [24] | High-purity, residue-free CO2 is essential to prevent contamination of extracts and system fouling. |
| Co-solvents | HPLC grade ethanol, methanol, acetone [24] [9] | Ethanol (food-grade) is preferred for pharmaceutical and nutraceutical applications due to low toxicity [9]. |
| Collection Solvents | HPLC grade 2-propanol, methanol, acetonitrile, hexane, chloroform [24] | Used to trap extracts post-depressurization in analytical SFE; selection depends on extract polarity. |
| Matrix Preparation | Freeze-dryer, laboratory mill [24] | Enables moisture removal and particle size reduction (~0.4-0.8 mm) to optimize mass transfer [24]. |
| Saponification Reagents | Potassium hydroxide, formic acid [24] | Used for post-processing of lipid extracts to isolate compounds like polyprenols from fatty acids [24]. |
| Analytical Standards | Compound-specific standards (e.g., polyprenol mixes) [24] | Essential for qualitative and quantitative analysis of target compounds via HPLC or LC-MS [24]. |
| 9,9-Bis(6-bromohexyl)fluorene | 9,9-Bis(6-bromohexyl)fluorene, CAS:269059-34-9, MF:C25H32Br2, MW:492.339 | Chemical Reagent |
| N-(2,2-dimethoxyethyl)prop-2-enamide | N-(2,2-dimethoxyethyl)prop-2-enamide, CAS:49707-23-5, MF:C7H13NO3, MW:159.18 g/mol | Chemical Reagent |
Advantages, Limitations, and Research Applications
Comparative Advantages
SFE-CO2 offers compelling advantages over conventional solvent extraction:
- Selectivity: Solvent properties can be fine-tuned by adjusting pressure and temperature, enabling selective extraction sequences [18].
- Speed: Diffusivities are much faster in supercritical fluids than liquids, and the lack of surface tension with low viscosity enables penetration into matrices inaccessible to liquids, reducing extraction time from hours to minutes [18] [16].
- Product Quality: Extraction occurs at moderate temperatures, protecting heat-sensitive compounds, and the process leaves no solvent residues in the final product [18] [9].
- Sustainability: CO2 is non-toxic, non-flammable, and can be recycled within the process, eliminating the use of petroleum-based solvents and reducing environmental impact [25] [9].
Technical and Economic Limitations
Despite its advantages, SFE-CO2 presents significant challenges:
- High Capital Cost: The requirement for high-pressure vessels, pumps, and pressure maintenance systems increases initial investment compared to conventional extraction [18] [9].
- Operational Complexity: Optimizing multiple interdependent parameters (pressure, temperature, flow, time) requires specialized expertise [9].
- Limited Polarity Range: Pure supercritical CO2 is non-polar and has limited effectiveness for highly polar compounds without modifiers [18] [9].
- Energy Intensity: Maintaining supercritical conditions is energy-intensive, particularly for large-scale systems [9].
- Scalability Challenges: Engineering large-scale systems that maintain control and extraction efficiency presents significant design hurdles [9].
Research and Industrial Applications
SFE-CO2 has demonstrated remarkable versatility across numerous research and industrial domains, including:
- Pharmaceutical/Nutraceutical Extraction: Isolation of bioactive compounds like polyprenols from plant materials (e.g., conifer species) with yields comparable to organic solvents but superior purity [24] [9].
- Food Industry: Decaffeination of coffee and tea, extraction of essential oils, spices, and colors, and removal of unwanted components (e.g., lipids) [18] [9].
- Environmental Applications: Cleaning and decontamination of precision components, medical devices, and recycled materials without generating hazardous solvent waste [25].
- Advanced Materials Processing: Aerogel drying, impregnation of polymers and textiles, and micronization of pharmaceutical compounds [25].
The following diagram illustrates the relationship between key process parameters and their effects on extraction performance:
The basic SFE-CO2 process represents a sophisticated yet highly controllable platform technology for sustainable extraction across multiple industries. From initial matrix preparation through pressurized extraction to final collection, each stage offers opportunities for optimization based on the specific properties of target compounds and the composition of the source matrix. While challenges remain in equipment cost and operational complexity, ongoing research continues to expand applications and improve efficiency. As industries increasingly prioritize green technologies and solvent-free products, SFE-CO2 stands positioned as a cornerstone technology for the future of separation science, particularly in pharmaceutical and nutraceutical development where purity, selectivity, and preservation of bioactivity are paramount.
SFE-CO2 Methods and Pharmaceutical Applications
Supercritical Carbon Dioxide (SC-CO₂) extraction represents a revolutionary advancement in separation technology, offering an environmentally friendly and efficient alternative to conventional solvent-based methods. This technology utilizes carbon dioxide above its critical temperature (304.12 K or 31.1 °C) and pressure (73.8 bar or 1071 psi), where it exhibits unique properties intermediate between gases and liquids [20] [26]. In this state, CO₂ possesses liquid-like densities with gas-like diffusivity and viscosity, enabling exceptional penetration into solid matrices and enhanced mass transfer rates [20]. The growing adoption of SC-CO₂ extraction across pharmaceuticals, food processing, and cosmetics underscores its significance in modern industrial applications, particularly as industries increasingly prioritize green chemistry principles [27] [20].
This technical guide provides an in-depth examination of three fundamental SC-COâ‚‚ extraction processes: Batch, Continuous Flow, and Dynamic extraction. Understanding the operational principles, applications, and comparative advantages of these methods is essential for researchers and drug development professionals seeking to implement supercritical fluid technology. The selectivity, efficiency, and sustainability of SC-COâ‚‚ extraction make it particularly valuable for processing thermolabile bioactive compounds, where preserving molecular integrity is paramount [28] [20].
Fundamental Principles of Supercritical COâ‚‚ Extraction
Supercritical CO₂ extraction operates on the principle of utilizing carbon dioxide in a state beyond its critical point, where it exhibits tunable solvent properties. The critical point of CO₂ is readily achievable (Tc = 31.1°C, Pc = 73.8 bar), making it practical for processing heat-sensitive compounds [20] [26]. In this supercritical state, CO₂ displays liquid-like solvation power while maintaining gas-like transport properties, with densities typically ranging from 0.3 to 0.9 g/cm³ [26].
The solvent power of SC-COâ‚‚ is primarily density-dependent and can be precisely controlled through manipulation of pressure and temperature parameters [20] [29]. This tunability enables selective extraction of target compounds by adjusting solvation characteristics. SC-COâ‚‚ is particularly effective for non-polar compounds; however, the addition of polar co-solvents (e.g., ethanol) can significantly enhance extraction efficiency for more polar molecules [27] [30] [20]. The basic SFE process comprises two fundamental stages: extraction (where SC-COâ‚‚ dissolves target compounds from the matrix) and separation (where pressure reduction causes solute precipitation) [20].
Table 1: Critical Properties of Common Substances for Supercritical Extraction
| Substance | Critical Temperature (°C) | Critical Pressure (bar) | Key Applications |
|---|---|---|---|
| Carbon Dioxide (COâ‚‚) | 31.1 | 73.8 | Universal solvent for non-polar to moderately polar compounds (essential oils, cannabinoids, antioxidants) |
| Water (Hâ‚‚O) | 374.0 | 220.6 | Extraction of polar compounds (rarely used due to harsh conditions) |
| Ethane (C₂H₆) | 32.2 | 48.8 | Alternative for non-polar compounds (limited use due to flammability) |
| Ethanol (Câ‚‚Hâ‚…OH) | 241.0 | 61.4 | Primarily used as polar co-solvent with SC-COâ‚‚ |
| Propane (C₃H₈) | 96.7 | 42.5 | Lipid extraction (limited use due to flammability risk) |
Core Extraction Methodologies
Batch Extraction
Principles and Operation: Batch extraction involves processing a fixed quantity of raw material in a closed system where the substrate remains stationary throughout the extraction cycle [27]. In this method, the extraction vessel is loaded with a specific amount of pre-treated biomass, sealed, and brought to the desired operating conditions. Supercritical COâ‚‚ is introduced and maintained in contact with the material for a predetermined period, allowing diffusion and solubilization of target compounds. Upon completion, the system is depressurized, and the extract is collected from the separation vessel [27] [29]. This approach is characterized by its operational simplicity and flexibility in handling diverse feedstocks.
Experimental Protocol for Batch Extraction:
- Sample Preparation: Biomass is typically dried (e.g., at 50°C for 48 hours) and milled to a specific particle size range (e.g., 0.25-0.50 mm) to enhance mass transfer while avoiding excessive channeling [31] [29].
- System Loading: The prepared material is packed into a tea bag or similar porous container and positioned in the extraction vessel to ensure even COâ‚‚ flow distribution [29].
- Pressurization and Heating: The system is sealed and pressurized with CO₂ using a syringe pump or compressor while temperature is adjusted to achieve supercritical conditions (typically 40-80°C, 150-450 bar) [30] [31].
- Static Extraction Phase: The supercritical COâ‚‚ remains in contact with the biomass for a specified static extraction period (e.g., 5-55 minutes) to allow saturation with solutes [30].
- Depressurization and Collection: The solute-laden COâ‚‚ is transferred to a separator where pressure reduction causes solute precipitation, and the extract is collected [29].
- System Purge: Residual COâ‚‚ is vented, and the spent biomass is removed [29].
Key Process Parameters:
- Particle size distribution (significantly impacts extraction kinetics) [31]
- Static extraction time (typically 5-55 minutes) [30]
- Temperature (40-80°C) and pressure (150-450 bar) [30]
- CO₂ to biomass ratio (e.g., ≥ 0.99 mass fraction) [29]
- Use of co-solvents (e.g., ethanol at 0-9% w/w) to enhance polarity [29]
Figure 1: Batch Extraction Workflow
Continuous Flow Extraction
Principles and Operation: Continuous flow extraction involves the steady passage of supercritical COâ‚‚ through an extraction chamber where raw material is continuously fed and extracted material is simultaneously removed [27]. This method maintains constant extraction conditions, allowing for uninterrupted operation and making it particularly suitable for large-scale industrial applications. The counter-current flow arrangement, where COâ‚‚ and biomass move in opposite directions, maximizes the concentration gradient driving force throughout the system, leading to enhanced extraction efficiency and higher throughput compared to batch systems [27].
Experimental Protocol for Continuous Flow Extraction:
- System Stabilization: The extraction system is brought to operational temperature and pressure before biomass introduction (typically 40-80°C, 150-450 bar) [30].
- Continuous Feeding: Prepared biomass is steadily fed into the extraction vessel using specialized high-pressure feeding mechanisms.
- Counter-current Operation: Supercritical COâ‚‚ is pumped through the system in the opposite direction to biomass flow, maximizing contact efficiency [27].
- Dynamic Extraction: The continuous flow of SC-COâ‚‚ through the biomass bed dissolves target compounds (dynamic extraction time typically 25-55 minutes) [30].
- Separation and Collection: The solute-rich COâ‚‚ stream passes through a pressure reduction valve into a separation chamber where extracts precipitate and are continuously collected.
- COâ‚‚ Recycling: The depressurized COâ‚‚ is condensed, re-pressurized, and returned to the extraction vessel in a closed-loop system [20].
Key Process Parameters:
- Solvent flow rate (significantly impacts extraction kinetics and specific consumption) [31]
- Biomass feed rate (determines residence time)
- Extractor diameter to length ratio (affects extraction rate and COâ‚‚ specific consumption) [31]
- Temperature (40-80°C) and pressure (150-450 bar) gradients along the system [30]
- Bed porosity and compaction (influence flow distribution and channeling) [31]
Figure 2: Continuous Flow Extraction System
Dynamic Extraction
Principles and Operation: Dynamic extraction combines elements of both batch and continuous processes, featuring periodic replenishment of raw material while maintaining a continuous flow of supercritical COâ‚‚ [27]. This hybrid approach allows for extended extraction times without interruption for vessel loading/unloading, striking a balance between operational efficiency and extraction effectiveness. The method is particularly advantageous for processes where raw material requires extended contact time with the solvent but where fully continuous feeding presents technical challenges [27].
Experimental Protocol for Dynamic Extraction:
- Initial System Charging: The extraction vessel is loaded with an initial batch of biomass.
- Continuous Solvent Flow: Supercritical CO₂ is continuously pumped through the system at predetermined conditions (e.g., 40-80°C, 150-450 bar) [30].
- Semi-continuous Operation: While COâ‚‚ flows continuously, biomass is periodically replenished either through multiple extraction vessels in parallel or specialized feeding systems.
- Extended Extraction Phases: The continuous COâ‚‚ flow is maintained for extended periods (dynamic extraction time typically 25-55 minutes per cycle) [30].
- Separation and Collection: Similar to continuous systems, solute-rich COâ‚‚ is expanded in separators for product recovery.
- Modular Operation: Multiple extraction vessels may be employed in tandem, allowing one vessel to be unloaded/loaded while others remain operational.
Key Process Parameters:
- Cycle time for biomass replenishment
- Dynamic extraction duration (typically 25-55 minutes) [30]
- COâ‚‚ flow rate (impacts extraction kinetics)
- Number of extraction vessels in parallel systems
- Temperature (40-80°C) and pressure (150-450 bar) stability during material transitions [30]
Comparative Analysis of Extraction Methods
Table 2: Comparative Analysis of SC-COâ‚‚ Extraction Methods
| Parameter | Batch Extraction | Continuous Flow Extraction | Dynamic Extraction |
|---|---|---|---|
| Process Scale | Laboratory to pilot scale (typically < 10L) | Large industrial scale | Pilot to medium industrial scale |
| Throughput | Low to medium | High | Medium to high |
| Operational Complexity | Low | High | Medium |
| Capital Cost | Low to medium | High | Medium to high |
| Flexibility | High (easy parameter changes) | Low (fixed parameters) | Medium |
| Optimal Applications | Research, high-value compounds, multiple small batches | Bulk commodities, single product lines | Medium-volume production, multiple similar compounds |
| Solvent Consumption | Higher per unit product | Lower per unit product | Intermediate |
| Yield Efficiency | Variable, often lower | High and consistent | Consistently high |
| Typical Extraction Time | 0.5 - 4 hours | Continuous operation | Extended cycles with periodic replenishment |
| Process Control | Simple | Complex | Moderately complex |
Advanced Technical Considerations
Process Optimization and Modeling
Optimizing SC-CO₂ extraction requires careful consideration of multiple interacting parameters. Mathematical modeling plays a crucial role in process design and scale-up, with the Sovová model being widely employed for simulating extraction kinetics from seed and plant materials [31]. This model accounts for both internal and external mass transfer limitations, distinguishing between "free" and "bound" solute in cellular structures. Recent advances incorporate machine learning approaches, with XGBoost algorithms demonstrating exceptional predictive capability for drug solubility in SC-CO₂ (R² = 0.9984, RMSE = 0.0605) [32].
Energy consumption analysis reveals significant nonlinearities in SC-COâ‚‚ processes, with regression models achieving Mean Absolute Percentage Errors of 7.6% in steady-state electricity consumption prediction [33]. Identification of dynamic energy consumption patterns enables real-time optimization strategies, particularly important for batch and dynamic systems where transient conditions prevail.
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Essential Research Reagents and Materials for SC-COâ‚‚ Extraction
| Reagent/Material | Function | Application Notes |
|---|---|---|
| Food-Grade COâ‚‚ (99.995%) | Primary supercritical solvent | Must be moisture-free; critical for all extraction types [29] [26] |
| Anhydrous Ethanol | Polar co-solvent | Enhances extraction of polar compounds (0-9% w/w typical) [30] [29] |
| Glass Beads/Pearls | Dispersant agent | Prevents selective channeling of COâ‚‚; improves yield [30] |
| Inert Ceramic Balls | Bed structuring | Improves flow distribution in continuous systems [31] |
| Silica Gel | Moisture scavenger | Protects system from moisture; critical for yield optimization [16] |
| Stainless Steel Porous Frits | Flow distribution | Ensures even COâ‚‚ flow through biomass beds [31] |
| High-Pressure Vessel Seals | System integrity | Material compatibility with COâ‚‚ essential (e.g., PTFE, special elastomers) |
| Analytical Reference Standards | Quantification | Essential for method validation and yield calculations |
| 9-Ethyldodecahydro-1H-carbazole | 9-Ethyldodecahydro-1H-carbazole, CAS:146900-30-3, MF:C14H25N, MW:207.35 g/mol | Chemical Reagent |
| 7-chloro-2H-benzo[e][1,2,4]thiadiazin-3(4H)-one 1,1-dioxide | 7-chloro-2H-benzo[e][1,2,4]thiadiazin-3(4H)-one 1,1-dioxide, CAS:5800-59-9, MF:C7H5ClN2O3S, MW:232.64 g/mol | Chemical Reagent |
Recent Technological Advances
Recent innovations in SC-CO₂ extraction have focused on enhancing selectivity, efficiency, and applicability to challenging matrices. Modifiers-assisted extraction utilizing ethanol, water, or other GRAS solvents significantly expands the polarity range of extractable compounds [27] [30]. Studies demonstrate that co-solvent implementation is the most significant factor (p < 0.05) for extracting bioactive metabolites from Arthrospira platensis, substantially improving yields of riboflavin, α-tocopherol, and β-carotene [30].
Fractionation techniques employing multiple separators in series enable selective recovery of different compound classes based on their unique solubility characteristics [27] [20]. This approach is particularly valuable for complex natural product extracts containing multiple valuable components with varying polarities.
Expanded bed extraction represents another innovation, increasing the contact surface area between COâ‚‚ and raw material by expanding the bed volume, thereby enhancing mass transfer and extraction efficiency, especially for materials with complex matrices [27].
Applications in Pharmaceutical and Natural Product Research
SC-CO₂ extraction has demonstrated exceptional utility in pharmaceutical and natural product research, particularly for thermolabile bioactive compounds. Research on Arthrospira platensis has shown that optimized SC-CO₂ extraction (450 bar, 60°C, 11 g/min ethanol co-solvent) effectively recovers functional extracts with significant antioxidant and antimicrobial activities [30]. The extracts obtained exhibited substantial bioactivity against clinically relevant pathogens including Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, and Candida albicans ATCC 10231 [30].
In drug development, SC-CO₂ extraction enables recovery of high-purity active pharmaceutical ingredients without solvent residues. Recent research has successfully extracted astaxanthin from engineered Corynebacterium glutamicum, achieving 93.3% recovery using optimized conditions (9% w/w ethanol, 68°C, 550 bar) [29]. The efficiency of SC-CO₂ extraction for intracellular compounds highlights its capability to penetrate cellular membranes while maintaining compound integrity.
Bibliometric analysis reveals a significant growth in SC-COâ‚‚ extraction research, with an annual publication increase of 8.79% and collaborative efforts spanning 42 countries, reflecting the method's expanding importance in pharmaceutical and natural product sciences [28].
Batch, continuous flow, and dynamic extraction methods each offer distinct advantages within the spectrum of supercritical COâ‚‚ extraction technologies. Batch processes provide maximum flexibility for research applications and small-scale production of high-value compounds. Continuous flow systems deliver unmatched efficiency and throughput for industrial-scale operations, while dynamic extraction represents a versatile intermediate approach suitable for pilot-scale development and medium-volume production.
The selection of an appropriate extraction methodology must consider multiple factors including target compound characteristics, scale requirements, economic constraints, and desired product quality. Ongoing advancements in process modeling, energy optimization, and hybrid approaches continue to expand the capabilities and applications of supercritical fluid technology across the pharmaceutical and natural products sectors. As green chemistry principles increasingly influence process development across industries, SC-COâ‚‚ extraction methodologies are poised for continued adoption and innovation.
Supercritical Carbon Dioxide (SC-CO₂) extraction has established itself as a cornerstone of green chemistry, providing an environmentally friendly alternative to conventional organic solvents. Its core principle utilizes carbon dioxide above its critical point (31.1 °C and 73.8 bar), where it exhibits unique liquid-like solvation power and gas-like diffusivity and viscosity [28] [20]. While the fundamentals of SC-CO₂ extraction are well-documented, advancing the technique's efficiency and selectivity requires sophisticated approaches. This guide details three advanced methodologies that push the boundaries of SC-CO₂ applications: fractionation, counter-current extraction, and modifiers-assisted extraction. These techniques enable researchers to tackle complex separation challenges, achieve higher purity extracts, and recover thermally labile or polar bioactive compounds with precision, thereby enhancing the scope of SC-CO₂ in pharmaceutical, nutraceutical, and food research [16] [20].
Core Principles and System Configuration
A standard SC-COâ‚‚ extraction system is comprised of several key components: a chiller for cooling COâ‚‚, a pump for pressurization and fluid delivery, an extraction vessel (or column) to hold the sample, an oven to maintain the system above COâ‚‚'s critical temperature, one or more separators for collecting the extract, and a back-pressure regulator to maintain system pressure [20]. The process can operate in dynamic mode, where SC-COâ‚‚ continuously flows through the sample, or static mode, where the fluid is held in contact with the sample for a set period before release [20].
The solvation power of SC-COâ‚‚ is highly tunable, primarily governed by its density, which is a function of temperature and pressure [26]. This tunability is the foundational principle behind advanced techniques. A moderate increase in pressure at constant temperature significantly increases fluid density, thereby enhancing its ability to dissolve target compounds [16]. The selection of advanced techniques depends on the research objective, as shown in Table 1.
Table 1: Guide to Selecting Advanced SFE Techniques
| Technique | Primary Research Objective | Key Mechanism | Ideal for Compound Types |
|---|---|---|---|
| Fractionation | Separate a complex extract into distinct fractions of different polarities or molecular weights. | Sequential or staged changes in pressure/temperature across multiple separators. | Mixtures of triglycerides, fatty acids, tocopherols, and sterols [16]. |
| Counter-Current Extraction | Continuous, high-efficiency separation of compounds with similar solubilities. | Continuous contact of ascending SC-COâ‚‚ with descending liquid feed in a packed column. | Concentrating minor components like tocopherols and sterols from deodorizer distillates [16]. |
| Modifiers-Assisted Extraction | Enhance yield and selectivity for polar bioactive compounds. | Addition of a small volume of polar co-solvent (e.g., ethanol) to SC-COâ‚‚ to modify its polarity. | Polyphenols, flavonoids, alkaloids, and other medium-to-high polarity molecules [24] [20]. |
Fractionation and Sequential Separation
Concept and Workflow
Fractionation refers to the process of separating a complex crude extract into two or more distinct fractions based on differences in compound solubility, molecular weight, or polarity. This is achieved by coupling multiple separators in series and manipulating the pressure and temperature in each vessel to create a solubility gradient [20]. As the SC-COâ‚‚ stream, laden with dissolved compounds, passes through successive separators, conditions are altered to cause specific compound classes to precipitate out in a controlled manner.
A typical workflow for a two-stage fractionation is illustrated below. This setup allows for the separation of a crude extract into a volatile/oily fraction and a less volatile/waxy fraction.
Experimental Protocol for Lipid Fractionation
The following protocol, adapted from industrial processes for deodorizer distillates, outlines a two-step fractionation to obtain a sterol-enriched fraction [16].
- Objective: To recover sterol-enriched triglyceride fractions from vegetable oil deodorizer distillate (DD).
- Sample Preparation: The DD is first chemically modified via esterification to convert free fatty acids (FFA) into fatty acid methyl esters (FAME), which increases the solubility of the oil matrix in SC-COâ‚‚ and improves subsequent separation efficiency [16].
- SC-COâ‚‚ System Setup: Configure the system with at least two separators in series.
- Extraction & Fractionation Steps:
- First Extraction Step: Load the esterified DD into the extraction vessel. Set the extraction pressure to 14 MPa and temperature to 45 °C. The SC-CO₂ will primarily dissolve and carry the FAME.
- First Separation Step: Direct the fluid to the first separator. Maintain similar conditions (e.g., 14 MPa, 45 °C). The FAME-rich fraction is collected here.
- Second Extraction Step: Continue the extraction, but now increase the pressure to 20 MPa and temperature to 80 °C. These more severe conditions will dissolve the less soluble, higher molecular weight compounds like sterols and tocopherols.
- Second Separation Step: Direct the fluid to the second separator. The sterol- and tocopherol-enriched fraction precipitates here for collection.
- Key Parameters: This method does not leave solvent residues, but the sterol fraction obtained may require an additional purification step to achieve high purity [16].
Counter-Current Supercritical Fluid Extraction
Concept and Workflow
Counter-current supercritical fluid extraction (CC-SFE) is a continuous and highly efficient contact method for separating liquid mixtures. In a CC-SFE column, the liquid feed (e.g., a pre-extracted oil) flows downward by gravity, while the SC-COâ‚‚ solvent flows upward. This continuous counter-current flow creates multiple equilibrium stages within a single column, allowing for highly selective separation of compounds with similar solubilities, such as concentrating tocopherols from fatty acid esters [16].
The process is visualized in the following diagram, showing the interaction between the liquid feed and the SC-COâ‚‚ solvent stream.
Experimental Protocol for Tocopherol Concentration
This protocol details the application of CC-SFE for concentrating tocopherols from chemically modified sunflower oil deodorizer distillate (SfODD) [16].
- Objective: To concentrate tocopherols in the raffinate stream by removing fatty acid ethyl esters (FAEE).
- Sample Pretreatment: Chemically modify the SfODD via esterification to transform the mixture into a system primarily containing tocopherols, sterols, and FAEE. A solid sterol fraction can be isolated as a by-product at this stage [16].
- CC-SFE System Setup: Utilize a system equipped with a continuous counter-current packed column.
- Extraction & Separation:
- Feed Introduction: Continuously introduce the pretreated, liquid SfODD mixture near the top of the column.
- SC-CO₂ Introduction: Introduce SC-CO₂ at the bottom of the column. The pressure and temperature are optimized, often in ranges of 180-300 bar and 40-60 °C [16].
- Counter-Current Contact: As the liquid feed descends, it encounters ascending SC-COâ‚‚. The FAEE, being more soluble, are preferentially extracted into the SC-COâ‚‚ stream.
- Product Collection:
- Extract (Top Stream): The SC-COâ‚‚, now rich in FAEE, exits the top of the column and is depressurized to recover the FAEE fraction.
- Raffinate (Bottom Stream): The remaining liquid, enriched in tocopherols and sterol esters due to the removal of FAEE, is collected from the bottom of the column.
- Key Parameters: The degree of tocopherol enrichment is highly sensitive to pressure and temperature. For example, one study achieved a ten-fold enrichment of tocopherols (from 4% to 36%) at 180 bar and 60 °C, as higher pressures can co-extract tocopherols and reduce raffinate purity [16].
Modifiers-Assisted Extraction
Concept and Modifier Selection
Supercritical COâ‚‚ is inherently non-polar, making it excellent for extracting lipids and essential oils but less effective for polar compounds. Modifiers (also called co-solvents) are small, precisely metered volumes of a polar solvent added to the main SC-COâ‚‚ stream to alter its polarity and solvation strength significantly [20]. Ethanol is the most prevalent modifier due to its safety, low toxicity, and status as a "green" solvent, making it suitable for food and pharmaceutical applications [24] [26]. Other solvents like methanol or water can be used for specific applications.
The primary functions of a modifier are to:
- Enhance the solubility of polar target molecules.
- Disrupt matrix-analyte interactions (e.g., hydrogen bonding) by interacting with the plant matrix, thereby improving mass transfer and extraction yield [20].
Experimental Protocol for Polyprenol Extraction
This protocol is based on optimized methods for extracting polyprenols from conifer species, demonstrating the critical impact of modifiers on yield [24].
- Objective: To maximize the extraction yield of polyprenols from Picea sitchensis needles using ethanol as a modifier.
- Sample Preparation: Freeze-dry and mill plant needles to a fine powder (~0.4–0.8 mm) to increase the surface area for extraction [24].
- SC-COâ‚‚ System Setup: Use a system equipped with a separate, precise co-solvent pump.
- Extraction & Separation:
- Load Sample: Place 3 g of powdered plant material into the extraction vessel.
- Set SC-CO₂ Parameters: Fix the CO₂ flow rate at 10 ml/min. Set the extraction pressure to 200 bar and temperature to 70 °C [24].
- Set Modifier Parameters: Set the ethanol co-solvent pump to a flow rate of 0.05 ml/min. This introduces approximately 0.5% ethanol by volume into the supercritical stream.
- Dynamic Extraction: Perform dynamic extraction for 7 hours.
- Separation & Collection: Depressurize the fluid stream into a separator to collect the polyprenol-rich extract.
- Key Parameters: The use of ethanol as a co-solvent under these conditions yielded 6.35 mg/g DW of polyprenol from P. sitchensis. This result underscores the necessity of modifiers for efficient extraction of certain medium-polarity bioactive compounds compared to pure SC-COâ‚‚ [24].
The Scientist's Toolkit: Research Reagent Solutions
Successful implementation of advanced SFE techniques requires specific reagents and materials. Table 2 lists essential items and their functions in the research process.
Table 2: Essential Research Reagents and Materials for Advanced SFE
| Reagent/Material | Function/Application | Technical Notes |
|---|---|---|
| Food/Grade Carbon Dioxide (COâ‚‚) | Primary supercritical solvent. | Purity > 99.9% is recommended to prevent clogging and contamination [24]. |
| Anhydrous Ethanol | Polar co-solvent (modifier). | Used to increase the polarity of SC-COâ‚‚ for extracting polyphenols, alkaloids, etc. [24] [26]. |
| Deodorizer Distillate (DD) | Model feedstock for fractionation and CC-SFE. | A complex mixture from vegetable oil refining, rich in FFA, tocopherols, and sterols [16]. |
| HP-5MS Capillary Column | GC-MS analysis for chemical composition of extracts. | Standard column for separating and identifying volatile and semi-volatile compounds [34]. |
| Polyprenol Standards (C70–C100) | Qualitative and quantitative analysis via HPLC. | External standards for calibrating and quantifying polyprenol homologs in extracts [24]. |
| Flash Chromatography System | Preliminary purification of complex SFE extracts. | Used for post-extraction purification of target compounds like polyprenols [24]. |
| Abrucomstat | Abrucomstat, CAS:100502-66-7, MF:C3H7NO4, MW:121.09 g/mol | Chemical Reagent |
| Irdye 700DX | Irdye 700DX, CAS:916821-46-0, MF:C74H96N12Na4O27S6Si3, MW:1954.2 g/mol | Chemical Reagent |
The advanced techniques of fractionation, counter-current extraction, and modifiers-assisted extraction significantly expand the capabilities of supercritical COâ‚‚ technology. By moving beyond simple batch extraction, researchers can achieve precise separations, enhance yields of high-value compounds, and develop more sustainable and efficient downstream processes. The integration of these methods, supported by robust experimental protocols and a deep understanding of thermodynamic principles, is paving the way for innovative applications in drug development, functional food creation, and the valorization of complex natural products. As the field evolves, the coupling of these advanced SFE techniques with cutting-edge analytical methods and process modeling will undoubtedly unlock new frontiers in green separation science.
The extraction and purification of bioactive compounds from natural sources is a fundamental and critical first step in harnessing their therapeutic potential for drug development. Natural products from medicinal plants, either as pure compounds or as standardized extracts, provide unlimited opportunities for new drug leads because of the unmatched availability of chemical diversity [35]. According to the World Health Organization (WHO), more than 80% of the world's population relies on traditional medicine for their primary healthcare needs, with plants used for traditional medicine containing a wide range of substances that can be used to treat chronic as well as infectious diseases [35]. The premier steps to utilize the biologically active compound from plant resources are extraction, pharmacological screening, isolation and characterization of bioactive compound, toxicological evaluation, and clinical evaluation [35].
Due to the development of adverse effects and microbial resistance to chemically synthesized drugs, scientific interest has turned to ethnopharmacognosy, discovering thousands of phytochemicals from plants as safe and broadly effective alternatives with less adverse effects [35]. Many beneficial biological activities such as anticancer, antimicrobial, antioxidant, antidiarrheal, analgesic, and wound healing activity have been reported from these compounds. However, the full potential of these bioactive compounds can only be realized through efficient, selective, and sustainable extraction and purification methodologies that preserve their chemical integrity and biological activity.
Supercritical Fluid Extraction with COâ‚‚: Fundamental Principles
The Supercritical State of Carbon Dioxide
Supercritical fluid extraction (SFE) using carbon dioxide represents one of the most significant advancements in extraction technology for bioactive compounds. A supercritical fluid is any substance existing above its critical point of temperature and pressure, where it exhibits unique properties intermediate between gases and liquids [26]. Carbon dioxide becomes supercritical when raised above its critical temperature of 31.1°C and critical pressure of 73.8 bar (1078 psi) [16] [26].
In this supercritical state, COâ‚‚ possesses several advantageous characteristics: it has low viscosity and high diffusivity similar to gases, enabling it to penetrate porous solid matrices effectively, while maintaining density and solvation power comparable to liquid organic solvents [16] [26]. Furthermore, its properties can be "tuned" by making small adjustments to temperature and pressure, allowing for selective extraction of target compounds [26]. Supercritical COâ‚‚ is also considered a "green" solvent due to its non-toxic, non-flammable nature, and low environmental impact as it is not classified as a volatile organic compound (VOC) [26].
Advantages Over Conventional Extraction Methods
Compared with traditional Soxhlet extraction, SFE uses supercritical fluid to provide a broad range of useful properties while eliminating the use of organic solvents, which reduces the problems of their storage, disposal, and environmental concerns [16]. In the extraction process, diffusion coefficients of lipids and waxes in supercritical fluids are much higher than in liquids, therefore extraction can occur more quickly [16]. Additionally, no surface tension is present in supercritical fluids, and viscosities are much lower than in liquids, which helps the supercritical fluids penetrate into small pores that are inaccessible to liquid solvents [16].
The advantages of SC-COâ‚‚ extraction are particularly evident when compared to conventional methods:
Table 1: Comparison of Extraction Techniques for Bioactive Compounds
| Extraction Method | Extraction Efficiency | Solvent Consumption | Processing Time | Thermal Degradation Risk | Environmental Impact |
|---|---|---|---|---|---|
| Supercritical COâ‚‚ | High to Very High [36] [37] | Low to Moderate [36] | Moderate [26] | Low [26] [37] | Low [36] [26] |
| Soxhlet Extraction | Moderate to High [35] | High [35] | Long (3-18 hours) [35] | Moderate to High [36] | High [36] |
| Ultrasound-Assisted | Moderate [37] | Moderate [36] | Short [36] | Low to Moderate [36] | Moderate [36] |
| Microwave-Assisted | Moderate to High [36] | Low to Moderate [36] | Short [36] | Moderate [36] | Moderate [36] |
| Maceration | Low to Moderate [35] | High [35] | Very Long (3-4 days) [35] | Low | High |
Optimization of Supercritical COâ‚‚ Extraction Parameters
Critical Process Parameters
The efficiency of SC-COâ‚‚ extraction depends on several interconnected parameters that must be optimized for each specific application and target compound. Recent research has systematically investigated these factors to maximize extraction yield, purity, and bioactivity preservation.
Pressure and Temperature: Pressure has a determining influence on extraction yield, with increasing pressure typically increasing the extraction rate by enhancing the solubility of target compounds [16]. Temperature also plays a significant role, though its effect is more complex due to competing influences on solute volatility and CO₂ density [24] [16]. Research on polyprenol extraction from conifers demonstrated that optimal yields were achieved at 200 bars pressure and 70°C temperature [24].
Extraction Time: The duration of dynamic extraction significantly impacts both yield and process economics. Studies on terpenoid extraction from Indocalamus latifolius leaves determined 4.5 hours as optimal [37], while polyprenol extraction research investigated dynamic times ranging from 70 minutes to 7 hours [24].
Cosolvent Modification: While supercritical COâ‚‚ is excellent for nonpolar compounds, its effectiveness for polar molecules can be enhanced through the addition of small percentages of polar cosolvents (typically 1-10%) such as ethanol, methanol, or water [26]. These modifiers alter the polarity of the supercritical fluid, expanding its application to a wider range of bioactive compounds. Research has shown that using absolute ethanol as a cosolvent with a flow rate of 0.05 ml minâ»Â¹ significantly improved polyprenol yields [24].
Systematic Optimization Approaches
Response surface methodology (RSM) with central composite design or Box-Behnken design has emerged as a powerful statistical approach for optimizing multiple SC-COâ‚‚ parameters simultaneously while understanding their interactive effects [37]. This methodology allows researchers to model the relationship between process variables and responses (yield, purity, bioactivity) with reduced experimental runs compared to one-factor-at-a-time approaches.
Table 2: Optimized SC-COâ‚‚ Parameters for Various Bioactive Compounds
| Bioactive Compound | Source Material | Optimal Pressure (bar) | Optimal Temperature (°C) | Optimal Time (min) | Cosolvent | Yield |
|---|---|---|---|---|---|---|
| Polyprenols | Picea sitchensis | 200 [24] | 70 [24] | 420 [24] | Ethanol | 6.35 ± 0.4 mg/g [24] |
| Terpenoids | Indocalamus latifolius | 260 [37] | 39 [37] | 270 [37] | Ethyl alcohol | 1.44 ± 0.12 mg/g [37] |
| Fatty Acids (EPA/DHA) | Nannochloropsis sp. | 100-550 [38] | 50-75 [38] | 100 [38] | Not specified | Varies with parameters [38] |
Experimental Protocols for SC-COâ‚‚ Extraction
Standardized Extraction Methodology
A typical SC-COâ‚‚ extraction process follows a systematic protocol that can be adapted based on specific research requirements and available equipment:
Sample Preparation: Plant materials should be pre-washed, freeze-dried, and ground to obtain a homogenous sample with particle size typically between 0.4-0.8 mm to improve mass transfer rates [24]. Proper actions must be taken to ensure that potential active constituents are not lost, distorted, or destroyed during sample preparation [35]. For some applications, biomass may be mixed with diatomaceous earth or glass beads to prevent caking and increase contact with COâ‚‚ [38].
Extraction Procedure: The general extraction workflow involves: (1) loading the prepared sample into the extraction vessel; (2) pressurizing the system with COâ‚‚ to the desired pressure while maintaining temperature; (3) dynamic extraction where supercritical COâ‚‚ continuously flows through the sample; (4) separation of extract from COâ‚‚ in a collection vessel through pressure reduction; and (5) collection and stabilization of the extract [24] [26]. The COâ‚‚ flow rate is typically maintained between 10-25 mL/min depending on the system scale [24] [38].
Extract Recovery: After extraction, the solute-laden COâ‚‚ is expanded to subcritical conditions through a pressure reduction valve or into a separate collection chamber, causing the extract to precipitate due to decreased solvation power [26]. The extract is then collected from the separation vessel, and any residual COâ‚‚ in the product naturally evaporates, leaving no solvent residues [26].
Analytical Characterization and Purification
Following extraction, comprehensive analysis is essential to characterize the chemical composition and biological activity of the obtained extracts:
Chromatographic Techniques: Thin-layer chromatography (TLC) provides a quick initial assessment of mixture complexity and can be coupled with bioautographic methods to localize antimicrobial components [35]. High-performance liquid chromatography (HPLC) represents the gold standard for isolation of natural products and is gaining popularity as the main choice for fingerprinting studies [35]. Gas chromatography-mass spectrometry (GC-MS) enables both qualitative and quantitative analysis of volatile and semi-volatile compounds, as demonstrated in terpenoid profiling from Indocalamus latifolius leaves [37].
Advanced Purification Methods: For obtaining pure bioactive compounds, subsequent purification steps employing techniques such as flash chromatography, WelFlash C18-l, BUCHI-C18, and Sephadex LH-20 chromatography can increase the purity of target compounds from 12.91% to over 93.34%, as demonstrated with terpenoid components [37].
Bioactivity Assessment: Validating the biological activity of extracts and purified compounds is essential through in vitro assays including antimicrobial screening, antioxidant activity (DPPH, ABTS, FRAP), cytotoxicity evaluation (MTT, CCK-8 assays), and specific mechanistic studies [39] [37].
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of SC-COâ‚‚ extraction and subsequent analysis requires specific laboratory reagents, materials, and equipment. The following table summarizes key components essential for research in this field:
Table 3: Essential Research Reagents and Materials for SC-COâ‚‚ Extraction Research
| Category | Specific Items | Function/Application | Examples from Literature |
|---|---|---|---|
| Extraction Solvents | Carbon dioxide (food grade, >99.9%) [24] | Primary supercritical fluid solvent | BOC (Limerick, Ireland) [24] |
| Cosolvents | Ethanol (≥99.8%), methanol, ethyl alcohol [24] [37] | Modifier for polar compound extraction | Absolute ethanol for polyprenol extraction [24] |
| Analytical Standards | Phytol, β-sitosterol, polyprenol mixes [24] [37] | Quantification and method calibration | LGC limited (Teddington, England) [24] |
| Chromatography Materials | WelFlash C18-l, BUCHI-C18, Sephadex LH-20 [37] | Purification and separation of compounds | Terpenoid purification [37] |
| Sample Preparation | Diatomaceous earth, glass beads (3 mm) [38] | Prevent biomass caking, improve COâ‚‚ contact | Microalgae extraction [38] |
| Analytical Instruments | GC-MS, HPLC, FTIR, scanning electron microscope [35] [37] | Compound identification and characterization | Shimadzu GCMS-QP2020 NX [37] |
| Propiophenone, alpha,alpha-dimethyl-beta-(dimethylamino)-, hydrochloride | Propiophenone, alpha,alpha-dimethyl-beta-(dimethylamino)-, hydrochloride, CAS:24206-69-7, MF:C13H20ClNO, MW:241.76 g/mol | Chemical Reagent | Bench Chemicals |
| N-Heptyl-1-naphthamide | N-Heptyl-1-naphthamide|High-Purity Research Chemical | Research-grade N-Heptyl-1-naphthamide, a corrosion inhibitor for acidic environments. This product is for research use only (RUO) and not for human consumption. | Bench Chemicals |
Applications in Drug Discovery and Development
Case Studies in Bioactive Compound Extraction
The application of SC-COâ‚‚ extraction has demonstrated significant success across various domains of natural product drug discovery:
Polyprenols from Coniferous Species: Research on Irish conifer species including Picea sitchensis, Cedrus atlantica 'Glauca', Pinus sylvestris, and Taxus baccata has established SC-CO₂ as an environmentally friendly alternative to organic solvent extraction for obtaining polyprenols, which exhibit non-carcinogenic, non-mutagenic, non-teratogenic, and non-toxic effects in humans while providing significant anti-tumour, anti-anaemia, anti-HIV, and anti-hepatitis C effects [24]. The optimized SC-CO₂ conditions (200 bars, 70°C, 7 hours, with ethanol cosolvent) yielded 6.35 ± 0.4 mg/g of polyprenols from P. sitchensis, demonstrating the method's efficacy [24].
Terpenoids from Medicinal Plants: Indocalamus latifolius leaf terpenoids extracted via SC-CO₂ demonstrated superior extraction rate, purity, and antioxidant activity compared to six conventional methods including steam distillation, ultra-high pressure-assisted, and ultrasound-assisted extraction [37]. The identified terpenoids (neophytadiene, phytol, β-sitosterol, β-amyrone, squalene, and friedelin) exhibited cytotoxicity against HepG2 cells with an IC₅₀ value of 148.93 ± 9.93 μg/mL and concentration-dependent cellular antioxidant activity [37].
Commercial Pharmaceutical Applications: Polyprenol-based products like ROPREN (highly purified mixture of polyprenols) and FORTEPREN (polyprenyl phosphate) represent successful commercial applications, marketed for liver disease treatment and as antiviral drugs with immunomodulating activity, respectively [24].
Integration with Downstream Processing
The effectiveness of SC-COâ‚‚ extraction must be considered within the broader context of downstream processing and drug development workflows. The integration with purification technologies such as countercurrent chromatography and preparative HPLC enables the production of compounds with pharmaceutical-grade purity [37]. Furthermore, the preservation of bioactivity through gentle SC-COâ‚‚ processing conditions (low temperatures, oxygen-free environment) ensures that extracted compounds maintain their therapeutic potential throughout the extraction and isolation pipeline [26] [37].
Supercritical COâ‚‚ extraction technology represents a paradigm shift in the approach to bioactive compound extraction for pharmaceutical applications. Its advantages include enhanced selectivity, greater purity of extracts, preservation of heat-sensitive compounds, and environmental sustainability [36] [37]. The integration of SC-COâ‚‚ extraction within the broader context of natural product drug discovery aligns with evolving regulatory expectations and consumer preferences for green technologies in pharmaceutical manufacturing.
Future developments in SC-COâ‚‚ extraction will likely focus on several key areas: (1) integration with continuous processing and Industry 4.0 technologies for improved process control and monitoring; (2) expansion to new natural sources including marine organisms, agricultural byproducts, and engineered biological systems; (3) hybrid approaches combining SC-COâ‚‚ with other green technologies such as microwave and ultrasound assistance; and (4) application to increasingly complex therapeutic compounds including proteins, peptides, and nucleic acids.
As research continues to demonstrate the technical and economic viability of SC-COâ‚‚ extraction for pharmaceutical applications, this technology is poised to become an increasingly central component of sustainable drug development pipelines. The ability to efficiently extract high-value bioactive compounds while minimizing environmental impact positions SC-COâ‚‚ technology as a key enabler for the next generation of natural product-derived therapeutics.
Supercritical fluid (SCF) technology, particularly using supercritical carbon dioxide (scCO₂), has emerged as a cornerstone of green and efficient particle engineering in pharmaceutical development. A supercritical fluid is defined as a substance at a temperature and pressure above its critical point, where it exhibits unique properties intermediate between those of a gas and a liquid. Specifically, scCO₂ possesses liquid-like densities that enable sufficient solvent power for many compounds, coupled with gas-like diffusivities and low viscosities that enhance mass transfer rates. The critical point of CO₂ is readily achievable (Tc = 31.1°C, Pc = 7.38 MPa), making it particularly suitable for processing thermolabile pharmaceutical compounds without degradation [40] [41].
The adoption of scCOâ‚‚-based technologies addresses significant limitations of conventional particle size reduction methods such as jet milling, ball milling, and spray drying. These traditional techniques often involve high shear forces, excessive temperatures, electrostatic charges, and abundant use of organic solvents, which can compromise product stability and necessitate costly purification steps [42]. In contrast, scCOâ‚‚ processes offer a sustainable alternative that is non-toxic, non-flammable, recyclable, and leaves minimal solvent residues [42] [43]. For the pharmaceutical industry, these technologies enable precise control over particle size, morphology, and crystal form, which are critical parameters for enhancing the bioavailability of poorly water-soluble drugs, a challenge affecting approximately two-thirds of pharmaceutical compounds [43].
This technical guide focuses on three principal supercritical fluid methods for particle engineering: Rapid Expansion of Supercritical Solutions (RESS), Supercritical Anti-Solvent (SAS), and Particles from Gas-Saturated Solutions (PGSS). These processes form the foundation of modern supercritical particle design within the broader context of supercritical COâ‚‚ extraction research, enabling the production of micronized and nano-sized drug particles with superior properties for advanced drug delivery applications.
Fundamental Principles and Comparative Analysis
Core Process Mechanisms
The RESS process leverages the tunable solvent power of scCOâ‚‚. A drug substance is first dissolved in scCOâ‚‚ within an extraction vessel at elevated pressure. This supercritical solution is then rapidly expanded through a nozzle into a low-pressure chamber. The drastic reduction in pressure causes an instantaneous decrease in solvent density and solvation capacity, leading to extreme supersaturation and the nucleation of fine particles. The entire process occurs in a single step without organic solvents, making it ideal for producing solvent-free, high-purity particles [42] [43].
In contrast, the SAS process employs scCOâ‚‚ as an anti-solvent rather than a solvent. The drug is first dissolved in a conventional organic solvent, and this solution is then sprayed as fine droplets into a vessel containing scCOâ‚‚. The scCOâ‚‚, which is miscible with the organic solvent but cannot dissolve the drug, rapidly diffuses into the droplets. This diffusion causes volume expansion and a reduction in solvent power, precipitating the drug as fine particles. The organic solvent is subsequently removed by a continuous flow of scCOâ‚‚ [40] [44]. The PGSS process differs from both RESS and SAS by focusing on the saturation of a molten or suspended substance with scCOâ‚‚. The drug substance, often a polymer, wax, or fat with a relatively low melting point, is melted in an autoclave. scCOâ‚‚ is then dissolved into this melt under pressure, creating a gas-saturated solution or suspension. When this mixture is depressurized through a nozzle, the rapid expansion of dissolved COâ‚‚ produces an intense cooling effect and atomizes the material into fine solid or hollow particles [45].
Table 1: Comparative Analysis of RESS, SAS, and PGSS Processes
| Feature | RESS | SAS | PGSS |
|---|---|---|---|
| Role of scCOâ‚‚ | Solvent | Anti-solvent | Solute / Propellant |
| Drug Requirement | Must be soluble in scCOâ‚‚ | Insoluble in scCOâ‚‚; soluble in organic solvent | Able to be melted or suspended; absorbs COâ‚‚ |
| Typical Particle Size | 50 nm - 5 μm [42] [43] | 50 nm - 10 μm [40] | 1 - 100 μm [45] |
| Key Advantage | No organic solvents; simple process | Handles polar, high MW drugs; controls polymorphism | Low energy requirement; high loading efficiency |
| Primary Limitation | Limited to scCOâ‚‚-soluble compounds | Requires organic solvent removal | Limited to meltable/suspendable materials |
The following workflow diagram illustrates the fundamental operational steps and logical progression common to these supercritical fluid processes, highlighting both their shared principles and key differentiators.
Figure 1: Generalized workflow for selecting and implementing supercritical fluid particle engineering techniques, showing the decision points based on drug substance properties.
Detailed Methodological Protocols
Rapid Expansion of Supercritical Solutions (RESS)
The RESS apparatus consists of a high-pressure CO₂ supply, a preheating coil, an extraction vessel where the drug dissolves in scCO₂, a nozzle for expansion (capillary or orifice disks with diameters typically 25-100 μm), and a particle collection chamber [42] [43]. The specific experimental protocol involves the following steps:
- Solubility Measurement: Determine the solubility of the target drug in scCOâ‚‚ across a range of temperatures (e.g., 40-80°C) and pressures (e.g., 10-30 MPa). This is a critical pre-screening step, as sufficient solubility (typically >10â»âµ in mole fraction) is required for successful processing [42].
- System Pressurization and Heating: Place the drug in the extraction vessel. Pressurize the system with COâ‚‚ to the desired operating pressure using a high-pressure pump and heat the entire system to the target temperature using thermostatic controls.
- Equilibration: Allow the system to equilibrate with continuous scCOâ‚‚ flow for a sufficient duration (e.g., 30-60 minutes) to ensure a saturated solution is achieved at the vessel outlet.
- Rapid Expansion: Expand the supercritical solution through the nozzle into the low-pressure collection chamber (maintained at ambient pressure or a carefully controlled back-pressure). The pre-expansion temperature, nozzle geometry, and spray distance are key parameters influencing particle size and morphology [42] [43].
- Particle Collection and Analysis: Collect the precipitated particles from the collection chamber and analyze them for size distribution (e.g., by laser diffraction), morphology (e.g., by scanning electron microscopy), and crystallinity (e.g., by X-ray diffraction).
The RESS process has been successfully applied to drugs like ibuprofen, diclofenac, and raloxifene, reducing their particle sizes to the nanometer range (e.g., 19 nm for raloxifene) and achieving significant dissolution rate enhancements [43].
Supercritical Anti-Solvent (SAS)
The SAS system comprises a high-pressure precipitation vessel (typically 100-500 mL), pumps for both the COâ‚‚ and the liquid drug solution, an injection nozzle (often coaxial for enhanced mixing), and a filtration system for particle collection [40] [44]. A detailed protocol is as follows:
- Solvent Selection: Dissolve the drug in a suitable organic solvent, such as acetone, methanol, or dimethyl sulfoxide, at a concentration of 1-5% w/v.
- Vessel Pressurization: Fill the precipitation vessel with scCO₂ using a high-pressure pump until the desired operating pressure (typically 8-15 MPa) and temperature (typically 35-60°C) are stably maintained.
- Solution Injection and Atomization: Inject the drug solution through the nozzle into the vessel as a fine spray. The scCOâ‚‚ acts as an anti-solvent, rapidly diffusing into the droplets and causing supersaturation and particle precipitation.
- Washing Phase: After the solution injection is complete, continue pumping pure scCOâ‚‚ through the vessel for a set duration (e.g., 30-90 minutes) to remove residual organic solvent from the precipitated particles.
- Depressurization and Collection: Slowly depressurize the vessel and collect the dry, solvent-free powder. The yield is determined by weighing the collected solid.
Variations of the SAS process include the Solution Enhanced Dispersion by Supercritical Fluids (SEDS) technique, which uses a specially designed coaxial nozzle to achieve intense mixing of the solution and scCOâ‚‚ simultaneously, leading to improved control over particle size distribution [44]. This method is highly effective for processing proteins, peptides, and other polar drugs that are insoluble in scCOâ‚‚.
Particles from Gas Saturated Solutions (PGSS)
The PGSS setup includes an autoclave or mixing vessel equipped with a stirrer and temperature control, a high-pressure pump for COâ‚‚, and a nozzle for depressurization. The procedural steps are:
- Melting/Suspension: Place the drug substance (or a drug-polymer mixture for composites) into the autoclave and heat it above its melting point or glass transition temperature. For solid suspensions, create a homogeneous suspension in a suitable medium.
- Saturation with COâ‚‚: Introduce scCOâ‚‚ into the autoclave under pressure and with constant agitation. The operating pressure typically ranges from 5 to 25 MPa. Allow sufficient time for the COâ‚‚ to saturate the melt/suspension, which can significantly lower its viscosity.
- Expansion and Atomization: Pump the gas-saturated solution through a nozzle into a collection chamber at atmospheric pressure. The rapid release of dissolved COâ‚‚ causes intense cooling (due to the Joule-Thomson effect) and breaks the material into fine droplets or solid particles.
- Particle Recovery: Collect the particles from the collection chamber. The low process temperature during expansion makes PGSS particularly suitable for heat-sensitive materials [45].
PGSS is highly effective for creating composite particles and microspheres for controlled drug release, as it allows for the efficient encapsulation of active ingredients within a polymeric matrix [43] [45].
Advanced Applications and Computational Modeling
Pharmaceutical Applications and Performance Data
Supercritical fluid technologies have demonstrated remarkable success in enhancing the bioavailability and performance of poorly water-soluble drugs (BCS Class II and IV). The following table summarizes quantitative data from various studies, illustrating the impact of these methods on key pharmaceutical properties.
Table 2: Experimental Performance Data of Drugs Processed by SCF Methods
| Drug | SCF Method | Key Process Conditions | Particle Size Result | Performance Outcome | Source |
|---|---|---|---|---|---|
| Cefuroxime Axetil | RESS | Not specified | 158 - 513 nm | >90% dissolution in 3 min vs. 50% in 60 min for commercial form | [43] |
| Raloxifene | RESS | 50°C, 17.7 MPa, 10 cm spray distance | 19 nm (from 45 μm) | 7-fold increase in dissolution rate | [43] |
| Diclofenac | RESS | Optimized parameters | 1.33 - 10.92 μm | Morphology change to quasi-spherical | [43] |
| Artemisinin | SAS / RESS | Not specified | Significant reduction | Improved dissolution rate | [43] |
| Ibuprofen (S-form) | RESS / Extraction | Not specified | Micronized | Enhanced chiral separation for targeted therapy | [41] |
| Letrozole | SCF Processing | 308-338 K, 12.2-35.5 MPa | Not specified | Solubility in scCOâ‚‚ measured from 0.03 to 1.45 mole fraction | [46] |
Beyond simple micronization, these technologies enable advanced applications. The Super-stable Homogeneous Intermix Formulating Technology (SHIFT), based on SCF principles, has been used to create ultrastable, homogeneous dispersions of hydrophilic small molecules like Indocyanine Green (ICG) in hydrophobic lipid embolic agents. This formulation demonstrated superior stability and photothermal properties compared to crude emulsions, proving highly effective for fluorescence-guided surgical resection of hepatocellular carcinoma [40]. Similarly, the Super-table Pure-Nanomedicine Formulation Technology (SPFT) has been applied to produce nano-sized chemotherapeutic agents and antibiotics with improved solubility, bioavailability, and therapeutic efficacy [40].
Machine Learning for Solubility Prediction and Process Optimization
A significant challenge in RESS and other scCOâ‚‚ processes is the experimental determination of drug solubility in scCOâ‚‚, which is costly and time-consuming. Machine learning (ML) models have recently emerged as powerful tools to accurately predict solubility, thereby accelerating process design [32] [47] [46].
Recent studies have employed advanced ensemble models such as XGBoost, CatBoost, and LightGBM, along with bio-inspired optimization algorithms, to predict drug solubility in scCO₂ using input features like temperature (T), pressure (P), critical properties (Tc, Pc), acentric factor (ω), molecular weight (MW), and melting point (Tm). One study on 68 drugs reported that an XGBoost model achieved an exceptional R² value of 0.9984 and a root mean square error (RMSE) of 0.0605 [32]. Another ensemble framework combining XGBR, LGBR, and CATr optimized by the Hippopotamus Optimization Algorithm (HOA) achieved an R² of 0.9920 [47]. For the specific case of Letrozole, an AdaBoost-KNN model optimized with a Golden Eagle Optimizer yielded an R² of 0.9945 in predicting its solubility across various temperatures and pressures [46].
The following diagram illustrates the integrated workflow of combining machine learning predictions with experimental SCF processing for efficient particle design.
Figure 2: Integration of machine learning models for predicting drug solubility in scCOâ‚‚ to guide and optimize experimental particle engineering processes.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of RESS, SAS, and PGSS requires specific reagents, equipment, and analytical tools. The following table details the key components of a research toolkit for supercritical fluid particle engineering.
Table 3: Essential Research Reagents and Equipment for SCF Particle Engineering
| Category | Item | Specification / Examples | Primary Function |
|---|---|---|---|
| Supercritical Fluid | Carbon Dioxide (CO₂) | High purity (≥ 99.9%) | Primary solvent/anti-solvent/propellant medium |
| Co-solvents / Modifiers | Ethanol, Methanol | HPLC grade | Enhance solubility of polar compounds in scCOâ‚‚ (for RESS) |
| Organic Solvents (for SAS) | Acetone, Dichloromethane, DMSO | Analytical grade | Dissolve the drug substance prior to anti-solvent precipitation |
| Polymeric Carriers | PLGA, PLLA, PEG | Pharmaceutical grade | Form composite particles and microspheres (for PGSS and SAS) |
| High-Pressure Equipment | Pump (for COâ‚‚) | Syringe or diaphragm type, P > 40 MPa | Deliver and pressurize COâ‚‚ to supercritical conditions |
| Pump (for solution) | HPLC or precision syringe pump | Deliver drug solution at a constant rate (for SAS) | |
| Precipitation Vessel | High-pressure autoclave with sight glass, V = 100-1000 mL | Main chamber for particle formation | |
| Nozzle | Capillary (25-100 μm) or coaxial | Facilitate rapid expansion or solution atomization | |
| Analytical Instruments | Scanning Electron Microscope (SEM) | Characterize particle morphology and surface topography | |
| Laser Diffraction Particle Sizer | Measure particle size distribution | ||
| X-Ray Diffractometer (XRD) | Determine crystallinity and polymorphic form | ||
| High-Performance Liquid Chromatography (HPLC) | Analyze drug content, purity, and dissolution profiles | ||
| Tachykinin angatonist 1 | Tachykinin angatonist 1, MF:C24H35Cl2N5O3S, MW:544.5 g/mol | Chemical Reagent | Bench Chemicals |
RESS, SAS, and PGSS represent a mature yet continuously evolving suite of technologies for particle engineering in drug delivery. By leveraging the unique properties of supercritical carbon dioxide, these methods overcome the significant drawbacks of conventional micronization techniques, enabling the production of particles with precise control over size, morphology, and solid state. The integration of modern computational tools, particularly advanced machine learning models, is now paving the way for data-driven prediction of solubility and optimization of process parameters, thereby reducing the experimental burden and accelerating the development of novel pharmaceutical formulations. As the demand for delivering poorly soluble drugs continues to grow, these supercritical fluid technologies, firmly grounded in the principles of green chemistry, are poised to remain at the forefront of innovative drug development strategies.
Supercritical fluid extraction (SFE) using carbon dioxide (SC-COâ‚‚) represents a green technology that aligns with the principles of sustainable chemistry within the broader thesis of supercritical COâ‚‚ extraction research. While SC-COâ‚‚ excels at extracting non-polar compounds like lipids, its efficiency for polar bioactive compounds, such as phenolics, is limited due to the non-polar nature of COâ‚‚. This case study investigates the strategic modification of SC-COâ‚‚ with ethanol as a co-solvent to enhance the recovery of valuable bioactive compounds from hemp seed oil, a topic of significant interest for researchers and drug development professionals seeking to optimize nutraceutical and pharmaceutical extracts.
Optimized SFE Parameters for Hemp Seed Oil
The foundational step in enhancing bioactive recovery is the optimization of core SFE parameters. Research employing Response Surface Methodology (RSM) with a Box-Behnken Design (BBD) has identified critical parameters for maximizing hemp seed oil yield and quality [48] [49]. The effects of temperature, pressure, and time were systematically assessed to establish a baseline operational window.
Table 1: Optimized SFE Parameters for Hemp Seed Oil Extraction [48] [49]
| Parameter | Investigated Range | Optimized Condition for Maximum Oil Yield | Key Influence on Extraction |
|---|---|---|---|
| Pressure | 10 - 20 MPa | 20 MPa | Positive linear effect; increased pressure increases SC-COâ‚‚ density and oil solubility. The most significant parameter for yield. |
| Temperature | 30 - 60 °C | 50 °C | Complex effect; higher temperatures reduce CO₂ density but increase solute vapor pressure. |
| Time | 120 - 300 min | 244 min | Positive linear effect; longer duration allows for greater mass transfer. |
| COâ‚‚ Flow Rate | 0.25 kg/h (fixed) | 0.25 kg/h | Literature suggests higher flow rates (e.g., 150 g/min) significantly improve cannabinoid recovery and total yield in larger systems [50]. |
Under these optimized conditions—50°C, 20 MPa, for 244 minutes—a maximum oil yield of 28.83 g/100 g of fresh hemp seeds was achieved [48] [49]. Statistical analysis of the model confirmed that pressure had the most profound linear effect on oil extraction efficiency [48].
The Co-Solvent Effect: Enhancing Bioactive Recovery
The primary limitation of pure SC-COâ‚‚ is its poor selectivity for polar bioactive molecules. The introduction of a polar co-solvent, such as ethanol, modifies the polarity of the supercritical fluid, thereby increasing its capacity to solubilize a wider range of compounds [48] [51]. This study tested COâ‚‚ modified with different proportions of ethanol (2.5%, 5%, 10%, and 20%) under the previously optimized SFE conditions [48] [49].
Table 2: Impact of Ethanol Modification (10%) on Hemp Seed Oil Composition and Stability [48] [49]
| Response Metric | Base SC-COâ‚‚ (Optimized) | SC-COâ‚‚ + 10% Ethanol | Change (%) | Functional Significance |
|---|---|---|---|---|
| Oil Yield | 28.83 g/100 g | 30.13 g/100 g | +4.5% | Improved overall mass recovery |
| Total Phenolic Content (TPC) | Not Specified (Base) | 294.15 mg GAE/kg | Significant Increase | Major enhancement in antioxidant capacity |
| Total Tocopherols | Not Specified (Base) | 484.38 mg/kg | Significant Increase | Improved Vitamin E activity and oxidative stability |
| Oxidative Stability Index (OSI) | Not Specified (Base) | 28.01 hours | Significant Increase | Extended shelf-life and resistance to rancidity |
| Key Phenolic Compounds | ||||
| ‧ N-trans-caffeoyltyramine | Not Detected | 50.32 mg/kg | - | Potent antioxidant with various biological activities |
| ‧ Cannabisin A | Not Detected | 13.72 mg/kg | - | Lignanamide with documented bioactivity |
| ‧ Cannabisin B | Not Detected | 16.11 mg/kg | - | Lignanamide with documented bioactivity |
The addition of 10% ethanol was identified as the optimal proportion, significantly boosting the recovery of key bioactives without adversely affecting the oil's quality parameters or fatty acid profile [48] [49]. Notably, HPLC-DAD/ESI-MS² analysis identified 26 phenolic compounds in the ethanol-modified extract that were not detected in the oil extracted with pure SC-CO₂ [48].
Detailed Experimental Protocol
Sample Preparation and Base SFE Optimization
- Plant Material: Whole hemp seeds are crushed and sieved to a uniform particle size of 500 μm [48] [49].
- Baseline Extraction: Load the biomass into the SFE extraction vessel. Maintain a CO₂ flow rate of 0.25 kg/h. Using a Box-Behnken experimental design, vary pressure (10-20 MPa), temperature (30-60°C), and time (120-300 min) to determine the optimal conditions for oil yield [48] [49].
- Collection: The extract is collected in a separator where pressure is reduced to atmospheric, causing COâ‚‚ to gasify and leaving the oil behind [52].
Ethanol-Modified SFE
- Co-solvent Addition: After determining the optimal parameters for yield, repeat the extraction process at these conditions (e.g., 50°C, 20 MPa) while introducing anhydrous ethanol as a co-solvent at varying proportions (2.5%, 5%, 10%, 20%) using a dedicated co-solvent pump [48] [49].
- Extraction and Analysis: Conduct the extraction for the optimized time (244 min). Collect the oil and analyze it for yield, bioactive content (TPC, tocopherols via HPLC), phenolic profile (HPLC-DAD/ESI-MS²), and oxidative stability (OSI) [48].
Experimental workflow for optimizing ethanol-modified supercritical COâ‚‚ extraction of hemp seed oil.
Analytical and Bioactive Profiling
Comprehensive analysis is critical for validating the efficacy of the ethanol-modified process. The application of HPLC-DAD/ESI-MS² was pivotal in identifying and quantifying 26 phenolic compounds in the ethanol-modified oil that were absent in the base SFE oil [48]. The most abundant compounds identified were:
- N-trans-caffeoyltyramine: 50.32 mg/kg oil
- Cannabisin B: 16.11 mg/kg oil
- Cannabisin A: 13.72 mg/kg oil [48]
This detailed phenolic profiling confirms that ethanol modification selectively enhances the extraction of specific, high-value antioxidant and bioactive compounds, which are crucial for pharmaceutical and cosmetic applications [48].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents and Materials for SFE of Hemp Seed Oil
| Reagent/Material | Function in Experiment | Specifications / Notes |
|---|---|---|
| Supercritical COâ‚‚ | Primary extraction solvent | GRAS (Generally Recognized as Safe) status, 99% purity [52] [50]. |
| Anhydrous Ethanol | Polar co-solvent | Increases solubility of phenolic compounds. Food/pharmaceutical grade required for final product safety [48] [53]. |
| Hemp Seeds | Raw biomass | Standardized, hulled, and crushed to a defined particle size (e.g., 500 μm) for consistent extraction [48] [49]. |
| HPLC-MS Grade Solvents | Extract analysis | Methanol, formic acid for LC-MS mobile phase preparation [51]. |
| Cannabinoid & Phenolic Standards | Analytical quantification | Certified reference standards for compounds like CBD, THC, cannabisin A/B for instrument calibration [51] [52]. |
| Enzymes for Comparison | Alternative extraction research | Cellulase, pectinase, hemicellulase for aqueous enzymatic extraction studies [54]. |
This case study demonstrates that modifying supercritical COâ‚‚ with 10% ethanol is a highly effective strategy to transcend the limitations of pure SC-COâ‚‚ extraction. This optimized protocol not only achieves a high oil yield but, more importantly, produces a hemp seed oil significantly enriched in phenolic compounds, tocopherols, and oxidative stability. The resulting high-value oil has enhanced potential for use in nutraceuticals, cosmetics, and pharmaceuticals, contributing meaningfully to the body of knowledge on advanced supercritical COâ‚‚ extraction technologies.
Optimizing SFE-CO2 Efficiency and Yield for Drug Development
Supercritical Carbon Dioxide (scCO₂) extraction has emerged as a cornerstone green technology in research and industrial applications, from pharmaceuticals to natural product isolation. This process utilizes CO₂ above its critical point (31.1 °C and 7.38 MPa), where it exhibits unique properties: gas-like diffusivity and viscosity coupled with liquid-like density and solvating power [28] [14]. The core principle making scCO₂ extraction both powerful and tunable is the direct and often dramatic interdependence of its pressure, temperature, and density. Mastering the interplay between these parameters is not merely an operational concern but a fundamental requirement for designing efficient, selective, and reproducible extraction processes. For researchers and drug development professionals, this mastery enables precise control over solute solubility, extraction kinetics, and final extract composition, making it critical for applications ranging from bioactive compound recovery to pharmaceutical particle engineering [48] [32].
The Fundamental Interplay of Pressure, Temperature, and Density
In supercritical fluid technology, pressure, temperature, and density are not independent variables. Their relationship governs the solvation power of scCOâ‚‚, which is directly proportional to its density [14]. This relationship is non-linear and presents a unique window of tunability for researchers.
The Pressure-Density-Solubility Relationship
At a constant temperature, increasing the pressure of scCO₂ leads to a significant increase in its density. This higher density enhances the solvation power of the CO₂, thereby increasing the solubility of target compounds. As demonstrated in the naphthalene example, solubility can skyrocket from a negligible 0.1 wt% at 70 atm to a substantial 10 wt% at 300 atm (at a constant 50°C) [14]. This profound effect makes pressure the most powerful lever for controlling extraction yield in a supercritical process.
The Temperature-Density-Solubility Relationship
The effect of temperature is more complex due to its dualistic influence. At a constant pressure, increasing temperature decreases the density of COâ‚‚ (which should lower solubility) but simultaneously increases the vapor pressure of the target solute (which should increase solubility). The dominant effect depends on the pressure regime:
- In the High-Pressure Region (e.g., above ~250 bar), the fluid is highly dense, and the vapor pressure effect dominates. Thus, increasing temperature enhances solubility.
- In the Low-Pressure Region (closer to the critical point), the density effect dominates. Thus, increasing temperature decreases solubility due to the rapid drop in density [14].
This trade-off leads to the phenomenon of a crossover pressure, where solubility isotherms for different temperatures intersect. This crossover point is a critical consideration for method development, as it identifies a pressure where solubility is independent of a range of temperatures.
Table 1: Effect of Core Parameters on scCOâ‚‚ Properties and Process Outcomes
| Parameter | Effect on scCOâ‚‚ Density | Effect on Solute Solubility | Primary Research Application |
|---|---|---|---|
| Pressure Increase (at constant T) | Increases | Increases | Maximizing yield for a wide range of solutes [48] [14]. |
| Temperature Increase (at constant P, High-Pressure Region) | Slightly Decreases | Increases (vapor pressure dominates) | Extracting less soluble, higher molecular weight compounds [48]. |
| Temperature Increase (at constant P, Low-Pressure Region) | Decreases significantly | Decreases (density dominates) | Potentially improving selectivity for highly volatile components. |
Quantitative Data and Experimental Optimization
Controlled experimentation is vital for quantifying the interplay of these parameters for specific applications. The following data, synthesized from recent research, illustrates how these principles are applied in practice.
Case Study: Optimizing Hemp Seed Oil Extraction
A 2025 study on hemp seed oil employed Response Surface Methodology (RSM) to optimize supercritical COâ‚‚ extraction. The results clearly demonstrate the primary role of pressure and the complex role of temperature [48].
Table 2: Optimization of Hemp Seed Oil Yield Using scCOâ‚‚ [48]
| Extraction Parameter | Range Studied | Optimal Condition | Effect on Oil Yield |
|---|---|---|---|
| Pressure (MPa) | 10 - 20 | 20 MPa | Strongest positive effect; yield increased with pressure due to increased COâ‚‚ density. |
| Temperature (°C) | 30 - 60 | 50 °C | Negative quadratic effect; yield increased to an optimum then decreased. |
| Time (min) | 120 - 300 | 244 min | Positive linear effect, with a negative quadratic effect at longer times. |
| Key Outcome | The maximum yield of 28.83 g/100 g of fresh seeds was achieved at 20 MPa, 50°C, for 244 min. |
Case Study: Extracting Polyprenol from Conifers
Research on extracting the bioactive compound polyprenol from Irish conifers further highlights the need for parameter optimization. A Lâ‚₆ (4³) orthogonal array design was used to test pressure (100-350 bar), temperature (40-70°C), and dynamic time (40-70 min) [24]. The results confirmed that the optimal parameter set is highly system-dependent. For Picea sitchensis, the highest polyprenol yield (6.35 ± 0.4 mg gâ»Â¹ DW) was achieved not at the highest pressure, but at 200 bar and 70°C with a 7-hour dynamic time, and using absolute ethanol as a co-solvent [24]. This underscores that maximum density does not always equate to maximum yield, especially for polar molecules where temperature and co-solvents play a critical role.
Advanced Considerations and Protocols
Detailed Experimental Protocol: scCOâ‚‚ Extraction of Hemp Seed Oil
The following protocol is adapted from the RSM-optimized study to serve as a template for researchers [48].
1. Objective: To maximize the yield and bioactive compound content of hemp seed oil using scCOâ‚‚. 2. Materials & Reagents:
- Hemp Seeds: Cleaned, dried, and milled to a particle size of ~500 μm.
- CO₂: High-purity (≥99.9%) food-grade carbon dioxide.
- Co-solvent: Anhydrous ethanol (for modified extraction). 3. Equipment Setup: A standard supercritical COâ‚‚ extraction system comprising:
- COâ‚‚ storage tank and chiller.
- High-pressure pump for COâ‚‚.
- Co-solvent pump (for modified extractions).
- Thermostatted extraction vessel.
- Separator vessel with pressure control.
- Flow meter and collection port. 4. Pre-Extraction:
- Load the extraction vessel with a known mass of ground hemp seed.
- Bring the system to the desired operating temperature and pressure. 5. Extraction Procedure:
- Initiate the COâ‚‚ flow at a constant rate (e.g., 0.25 kg/h).
- For co-solvent experiments, introduce ethanol at a defined flow rate (e.g., 10% of COâ‚‚ flow).
- Maintain the dynamic extraction for the set time (e.g., 244 min).
- The extract is collected in the separator where pressure is reduced, causing the solute to precipitate. 6. Post-Extraction:
- Weigh the collected oil to determine yield.
- Analyze for target bioactive compounds (e.g., total phenols, tocopherols via HPLC).
The Role of Co-solvents and Pretreatment
The solubility of polar molecules can be limited in pure scCOâ‚‚. The addition of a small percentage (e.g., 2-10%) of a polar co-solvent, or modifier, like ethanol, can dramatically enhance the extraction efficiency of phenolic compounds and other polar bioactives by increasing the solvent's polarity and interacting with the plant matrix [48] [24]. For instance, adding 10% ethanol to scCOâ‚‚ for hemp seed oil extraction increased the total phenolic content (TPC) from a base level to 294.15 GAE mg/kg [48].
Pretreatment of the raw material is another key intensification strategy. Studies on Hetian rose essential oil showed that pretreating petals with salt or enzyme solutions can significantly improve the extraction rate by disrupting cell walls. A 10% salt solution pretreatment resulted in an 8.99% extraction rate, compared to 4.21% with a 20% salt solution, demonstrating that optimal concentration is critical [55].
Machine Learning for Solubility Prediction
Given the cost and time of experimental solubility measurement, machine learning (ML) has become a powerful tool. Recent research uses models like XGBoost, CatBoost, and Random Forest to predict drug solubility in scCO₂ based on inputs like temperature, pressure, CO₂ density, and drug properties (molecular weight, melting point) [32]. One study reported that an XGBoost model achieved an exceptional R² value of 0.9984, providing researchers with a highly accurate predictive tool to guide experimental design and reduce the parameter space that needs to be tested empirically [32].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for scCOâ‚‚ Extraction Research
| Item | Specification / Function | Research Application / Note |
|---|---|---|
| Carbon Dioxide | High-purity (≥99.9%), food or pharmaceutical grade. | The primary supercritical solvent. Purity is essential to avoid contamination [24] [55]. |
| Co-solvents | Ethanol (anhydrous), Methanol. HPLC or reagent grade. | Modifies the polarity of scCOâ‚‚ to enhance solubility of polar compounds (e.g., phenolics) [48]. |
| Enzymes | Cellulase, Pectinase (≥99% purity). | Used for enzymatic pretreatment of plant matrices to disrupt cell walls and improve mass transfer [55]. |
| Salt Solutions | Sodium Chloride (NaCl), ACS grade. | Used in salt solution pretreatment to create osmotic pressure, aiding in cell rupture [55]. |
| Reference Standards | Analytic standards of target compounds (e.g., polyprenol, drug compounds). | Essential for qualitative and quantitative analysis (e.g., via HPLC-MS) to validate extraction efficacy [24]. |
Process Visualization and Workflow
The following diagram illustrates the logical decision-making workflow and the feedback loop between parameter adjustment, core property changes, and experimental outcomes in scCOâ‚‚ extraction research.
The journey to mastering supercritical CO₂ extraction is fundamentally a journey of understanding the dynamic interplay between pressure, temperature, and density. As this guide has detailed, pressure serves as the primary driver for solubility, while temperature exerts a more nuanced, pressure-dependent influence. The advent of statistical optimization tools like RSM and predictive machine learning models provides researchers with an unprecedented ability to navigate this complex parameter space efficiently. Furthermore, the strategic use of co-solvents and matrix pretreatment methods extends the reach of scCO₂ to a wider range of valuable compounds. For scientists in drug development and beyond, a deep, practical command of these core parameters is the key to unlocking the full potential of this versatile, green technology—enabling the design of processes that are not only highly efficient and selective but also scalable and sustainable.
The Role of CO2 Flow Rate and Extraction Time in Process Efficiency
Supercritical carbon dioxide (SC-CO2) extraction has emerged as a green alternative to conventional solvent-based separation techniques, offering significant advantages for extracting sensitive bioactive compounds from natural materials [20]. The efficiency of this process is governed by a complex interplay of several parameters, with CO2 flow rate and extraction time representing two of the most critical operational factors that researchers and engineers must optimize [56] [57]. Within the broader thesis on the fundamentals of supercritical CO2 extraction research, understanding the role of these parameters is essential for developing efficient, scalable, and economically viable processes for the pharmaceutical, food, and nutraceutical industries.
This technical guide provides an in-depth examination of how flow rate and extraction time jointly influence mass transfer kinetics, solubility equilibrium, and overall process efficiency. We explore the underlying principles, present consolidated experimental data from recent studies, and detail protocols for systematic optimization, providing scientists and drug development professionals with a comprehensive framework for advancing their SC-CO2 extraction research.
Theoretical Foundations: Flow Rate and Time in Mass Transfer
The SC-CO2 extraction process can be conceptually divided into two main periods: a constant extraction rate (CER) period controlled by solubility equilibrium, followed by a diffusion-controlled period governed by internal mass transfer resistance [58] [56].
The Interplay of Flow Rate and Extraction Time
During the initial CER period, the solute concentration in the SC-CO2 stream remains at its saturation point. In this phase, increasing the CO2 flow rate typically enhances the extraction efficiency by delivering more fresh solvent to the plant matrix per unit time, thus increasing the mass transfer driving force [59]. However, this relationship is not linear indefinitely. Beyond an optimal point, excessively high flow rates can cause fluid channeling, where CO2 bypasses significant portions of the plant material, resulting in incomplete extraction and solvent wastage [56].
The transition to the diffusion-controlled period occurs when the easily accessible surface solute is depleted. The extraction rate then becomes limited by the diffusion speed of solutes from the interior of the plant matrix to the surface, making extraction time the dominant factor for achieving high cumulative yields [58]. The optimal flow rate must therefore ensure sufficient contact time for the CO2 to saturate with solute while minimizing the overall process time to achieve economic viability [59].
Figure 1: Mass Transfer Periods in Supercritical CO2 Extraction
Quantitative Data Analysis: Experimental Values Across Applications
The optimal combination of flow rate and extraction time varies significantly depending on the raw material characteristics, target compounds, and specific extraction system used. The following table consolidates experimental data from recent research studies, providing a reference for developing new extraction protocols.
Table 1: Experimental CO2 Flow Rate and Extraction Time Parameters Across Different Applications
| Source Material | Target Compound | CO2 Flow Rate | Extraction Time | Optimal Yield | Pressure & Temperature | Reference |
|---|---|---|---|---|---|---|
| Zanthoxylum schinifolium | Essential Oil | 18 L/h (~0.3 L/min) | 1.5 hours | 9.40% | 14 MPa, 55°C | [60] |
| Haematococcus pluvialis | Astaxanthin | 2 L/min | 175 min | 95% recovery | 50 MPa, 50°C | [58] |
| Haematococcus pluvialis | Astaxanthin | 4 L/min | 95 min | 95% recovery | 50 MPa, 50°C | [58] |
| Grapefruit (Citrus paradisi) | Lycopene | 35 g/min | 135 min | Maximum yield | 305 bar, 70°C | [21] |
| Cannabis flowers | Cannabinoids (THC, CBG) | 15 g/min | 2 hours | 64.3 g THC/100g | 235 bar, 55°C | [52] |
| Cannabis flowers | Cannabinoids (CBN) | 15 g/min | 4 hours | 2.4 g CBN/100g | 235 bar, 55°C | [52] |
The data demonstrates that higher flow rates can substantially reduce extraction time while achieving similar yields, as evidenced by the astaxanthin extraction where doubling the flow rate from 2 L/min to 4 L/min reduced the required time from 175 to 95 minutes for equivalent recovery [58]. Similarly, different cannabinoids required varying extraction times at the same flow rate, with CBN needing significantly longer extraction periods than THC or CBG [52].
Table 2: Effect of CO2 Flow Rate on Extraction Efficiency Metrics
| Flow Rate Characteristic | Impact on Extraction Kinetics | Effect on Solvent Consumption | Process Economics |
|---|---|---|---|
| Low Flow Rate (<1-2 L/min for lab scale) | Longer saturation time, increased risk of diffusion limitation | Lower total CO2 consumption per batch | Higher energy and time costs per product mass |
| Moderate Flow Rate (2-4 L/min for lab scale) | Balanced saturation and mass transfer | Moderate CO2 consumption with good efficiency | Often represents optimal economic balance |
| High Flow Rate (>4 L/min for lab scale) | Reduced contact time, potential channeling | Higher instantaneous CO2 consumption, faster processing | Lower time-related costs, potential solvent recycling challenges |
| Variable/Programmed Flow | Dynamic adjustment to extraction phases | Potentially optimized total consumption | Requires advanced control systems but can maximize efficiency |
Experimental Protocols and Optimization Methodologies
Systematic Optimization Using Response Surface Methodology
Response Surface Methodology (RSM) provides a statistically rigorous framework for optimizing flow rate and extraction time while accounting for their interactions with other parameters like pressure and temperature [60] [57]. The following protocol outlines a standardized approach for method development:
Experimental Design: Employ a Central Composite Design (CCD) or Box-Behnken Design with flow rate and extraction time as independent variables, with yield and/or purity as response variables [21] [52]. Include at least 3-5 levels for each factor to adequately model curvature in the response surface.
Parameter Ranges: Based on published studies, appropriate ranges for initial screening are:
Model Development: Conduct experiments according to the design matrix and fit a second-order polynomial model to the data. The model should include linear, quadratic, and interaction terms for all factors [21].
Optimization and Validation: Use the fitted model to identify optimal parameter combinations, then perform confirmation experiments to verify predictions. For example, one study identified 18 L/h flow rate with 1.5 hours extraction time as optimal for Zanthoxylum schinifolium essential oil [60].
Protocol: Kinetics Study for Flow Rate and Time Optimization
This protocol determines the optimal flow rate and extraction time for a new plant material by establishing the extraction kinetics profile.
Materials and Equipment:
- Supercritical fluid extraction system with flow control [59]
- Analytical balance (±0.0001 g)
- Raw plant material (properly pretreated)
- CO2 supply (high purity, >99.5%)
Procedure:
- Sample Preparation: Prepare plant material by drying and grinding to uniform particle size (typically 0.3-0.5 mm). Precisely weigh batches (e.g., 100 g) for each experimental run [58].
Fixed-Parameter Setup: Set constant extraction pressure and temperature based on preliminary tests or literature values for similar materials.
Flow Rate Series: Conduct extractions at different CO2 flow rates (e.g., 1, 2, 3, 4 L/min) while maintaining constant pressure, temperature, and total extraction time.
Time-Course Monitoring: For each flow rate, collect extract fractions at regular time intervals (e.g., every 30 minutes) and weigh accurately to establish the extraction kinetics profile.
Analysis: Quantify target compounds in each fraction using appropriate analytical methods (GC-MS, HPLC, etc.).
Data Processing: Calculate cumulative yield versus time for each flow rate. Plot extraction curves and determine the time where the curve plateaus for each flow rate.
Interpretation: The optimal flow rate provides the shortest time to reach yield plateau without excessive CO2 consumption. The optimal extraction time is the point where the increase in yield becomes negligible with additional time (diminishing returns) [58] [56].
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Research Reagents and Materials for SC-CO2 Extraction Studies
| Reagent/Material | Function in Research | Technical Specifications | Application Notes |
|---|---|---|---|
| Supercritical CO2 | Primary extraction solvent | High purity (>99.5%), often with dip tube for liquid withdrawal | Critical for reproducible results; lower purity may contain moisture affecting phase behavior [21] [52] |
| Co-solvents (e.g., Ethanol) | Modifier for polar compounds | HPLC or food grade, anhydrous (≥99.5%) | Typically added at 1-15% to enhance polarity; must be accounted for in mass balance calculations [21] [52] |
| Reference Standards | Quantification and method validation | Certified reference materials with documented purity | Essential for developing accurate analytical methods; should include target compounds and possible degradation products [52] |
| Cell Disruption Aids | Enhance access to intracellular compounds | Glass beads, sand, or other inert disruption media | Particularly important for materials with tough cell walls like microalgae or seeds [58] |
| Analytical Solvents | Extract collection and analysis | HPLC grade solvents (methanol, hexane, etc.) | Should be selected for compatibility with downstream analysis methods [21] |
CO2 flow rate and extraction time represent interdependent parameters that collectively determine the efficiency and economic feasibility of supercritical fluid extraction processes. The optimal combination of these factors is highly specific to the raw material characteristics, target compounds, and equipment configuration. Through systematic optimization approaches like Response Surface Methodology and detailed kinetics studies, researchers can identify conditions that maximize yield and selectivity while minimizing processing time and solvent consumption. As SC-CO2 extraction continues to evolve in pharmaceutical and nutraceutical applications, advanced control strategies that dynamically adjust flow rates throughout the extraction process may offer further enhancements in process efficiency and product quality.
Raw material preparation is a critical first step in supercritical carbon dioxide (ScCO2) extraction, directly influencing the mass transfer efficiency, extraction yield, and final product quality. The physicochemical properties of ScCO2—density, viscosity, and diffusivity—are highly dependent on temperature and pressure, but their effectiveness in penetrating the solid matrix and solubilizing target compounds is predominantly governed by the physical characteristics of the raw material itself [61]. Among these characteristics, particle size and moisture content are two of the most significant and controllable factors. This guide provides an in-depth examination of their impacts, supported by quantitative data, experimental protocols, and practical insights for researchers and development professionals.
The Impact of Particle Size
Fundamental Principles and Mechanisms
Particle size reduction is a common pretreatment in ScCO2 extraction because it directly enhances the accessibility of the supercritical fluid to the target compounds. The underlying mechanisms are grounded in fundamental principles of mass transfer and surface area.
- Increased Surface Area: Reducing particle size increases the specific surface area exposed to ScCO2, thereby providing more contact points for the solvent to interact with the solute [62].
- Shortened Diffusion Path: A smaller particle size shortens the internal diffusion path length that the solute must travel to reach the particle surface and be carried away by the bulk fluid [63].
- Enhanced Disruption of Cellular Structures: Grinding can rupture the cell walls that contain the desired compounds, transforming intact cells into "broken cells." This allows for faster convective mass transfer of the solute, as opposed to the slower diffusion process that dominates in intact cells [63].
Quantitative Data on Particle Size Effects
The following table summarizes empirical findings on the effect of particle size from recent research.
Table 1: Quantitative Effects of Particle Size on ScCOâ‚‚ Extraction Efficiency
| Raw Material | Particle Size Range | Key Findings | Source |
|---|---|---|---|
| Roselle (Hibiscus sabdariffa) | 250–355 µm | Highest recovery: 11.96% global yield, 42.93 mg/100 g TPC, and 239.36 mg/100 g TFC. Highest solubility of global yield (1.50 g/L). | [62] |
| 355–425 µm | Intermediate recovery and solubility values. | ||
| 425–500 µm | Lowest recovery and solubility values. | ||
| Shale | 0.074–0.2 mm | Greatest increase in total pore volume and specific surface area after ScCO₂ treatment, enhancing gas accessibility. | [64] |
| 0.2–0.25 mm | Moderate impact on pore structure. | ||
| 1–3 mm | Limited effect on the internal pore-fracture network. | ||
| Pistacia lentiscus L. | 220 µm | Higher extraction yield achieved compared to larger particles. | [63] |
| 650 µm | Lower extraction yield. |
Experimental Protocol: Determining Optimal Particle Size
Objective: To determine the optimal particle size for the ScCO2 extraction of bioactive compounds from a plant material.
Materials and Equipment:
- Raw plant material (e.g., dried roselle calyces)
- Professional grinder (e.g., Panasonic blender)
- Digital sieve shaker (e.g., Endecott's Octagon 2000) with a set of sieves
- Analytical balance
- ScCO2 extraction system (e.g., 50 mL extraction vessel, CO2 pump, back-pressure regulator)
Methodology:
- Preparation and Grinding: Dry the raw plant material uniformly to a low moisture content (e.g., below 8%). Use a professional grinder to comminute the material.
- Sieving and Classification: Use a digital sieve shaker to separate the ground material into distinct, well-defined particle size fractions. Common fractions used in research include:
- 250 µm < dp < 355 µm
- 355 µm < dp < 425 µm
- 425 µm < dp < 500 µm [62]
- Extraction Experiment: Load a fixed mass (e.g., 3 ± 0.005 g) of each particle size fraction into the ScCO2 extraction vessel. Maintain constant extraction parameters (e.g., pressure, temperature, CO2 flow rate, time, and co-solvent percentage) across all runs to isolate the effect of particle size.
- Analysis and Comparison: Weigh the extracted oil to determine the global yield. Analyze the extracts for specific target compounds (e.g., Total Phenolic Content, Total Anthocyanin Content) using appropriate analytical techniques (e.g., UV-Vis spectrophotometry, HPLC). Compare the yields and compositions across the different particle size fractions to identify the optimum.
The Impact of Moisture Content
Fundamental Principles and Competing Effects
The role of moisture in ScCO2 extraction is complex and can lead to opposing effects, making its optimization highly matrix-specific.
Negative Impacts (Hindrance):
- Pore Blocking: Water can occupy pores and interact with the matrix through hydrogen bonding, creating a physical barrier that blocks the access of ScCO2 to the target compounds and impedes the diffusion of solutes [65].
- Competitive Solvation: Water is a polar molecule and can compete with ScCO2 for polar solute molecules, potentially reducing the extraction efficiency of certain compounds.
Positive Impacts (Enhancement):
- Matrix Swelling: The presence of moisture can cause the plant matrix to swell, thereby opening up the internal structure and creating new channels for ScCO2 to penetrate. This can improve the overall permeability of the matrix [65].
- Enhanced Hydrolysis: In some cases, water can act as a reactant or catalyst, facilitating the hydrolysis of compounds and freeing bound analytes.
Quantitative Data on Moisture Content Effects
Table 2: Quantitative Effects of Moisture Content on ScCOâ‚‚ Extraction Efficiency
| Raw Material | Moisture Content Range | Key Findings | Source |
|---|---|---|---|
| Hermetia illucens Larvae | 4.45–15.95% w/w | No negative effect on oil recovery efficiency was observed in this range. Recovery was primarily determined by the initial oil content of the larvae. | [65] |
| No significant differences in the fatty acid profile of the extracted oils. | |||
| Moisture content in the final oil ranged from 0.118–1.706% w/w, with some samples exceeding the recommended 0.2% for volatile matter in edible oils. |
Experimental Protocol: Standardizing and Modifying Moisture Content
Objective: To evaluate the effect of the raw material's initial moisture content on ScCO2 extraction yield and quality.
Materials and Equipment:
- Raw material (e.g., insect larvae, plant matter)
- Oven or freeze-dryer
- Analytical balance
- Desiccators
- Humidity-controlled chamber (optional)
Methodology:
- Baseline Moisture Determination: Determine the initial moisture content of the raw material gravimetrically by drying a representative sample (e.g., 2 g) in an oven at 105°C until a constant weight is achieved [65]. The moisture content is calculated from the weight loss.
- Moisture Standardization/Modification:
- To Reduce Moisture: Dry the bulk raw material in a controlled oven or via freeze-drying to the desired lower moisture level.
- To Increase Moisture: Add a calculated amount of distilled water to the dried material, mix thoroughly, and store in a sealed container at 4°C for several hours or days to allow for uniform water distribution.
- Extraction and Analysis: Conduct ScCO2 extraction on batches with varying, precisely known moisture contents while keeping all other parameters (particle size, pressure, temperature) constant. Analyze the extraction yield and, if applicable, the quality of the extract (e.g., peroxide value, compound profile).
Integrated Workflow and Practical Considerations
The preparation of raw material is a multi-step process where particle size and moisture content are interconnected. The diagram below illustrates a logical workflow for systematic raw material preparation.
Diagram Title: Raw Material Prep Workflow
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials and Reagents for Raw Material Preparation Research
| Item | Function/Application | Example from Literature |
|---|---|---|
| Professional Grinder | To achieve uniform and controllable particle size reduction. | Panasonic professional blender [62]. |
| Digital Sieve Shaker | To separate the ground material into precise, narrow particle size distributions for controlled experiments. | Endecott’s Octagon 2000 Digital Sieve Shaker [62]. |
| Laboratory Oven | For drying raw materials to a specific, low moisture content prior to grinding and extraction. | Drying at 105°C for moisture content determination [65]. |
| Analytical Balance | For precise weighing of raw materials and extracts to calculate yields and moisture content. | Used for gravimetric analysis [65] [52]. |
| Ethanol (Analytical Grade) | Acts as a polar co-solvent to modify ScCO2, improving the extraction efficiency of medium-polarity compounds like phenolics. | Used at 0.24 mL/min as an entrainer in roselle extraction [62]; 10% ethanol in hemp seed oil extraction [48]. |
| Carbon Dioxide (High Purity) | The primary supercritical solvent. Purity ≥99.5% is typically required to avoid contamination. | CO2 with 99% purity [62]; N-38 grade CO2 [65]. |
The preparation of raw materials is a foundational step that can dictate the success of a supercritical CO2 extraction process. As demonstrated by empirical data, reducing particle size consistently enhances extraction yield and kinetics by improving mass transfer parameters. The effect of moisture content is more nuanced but can be managed; a moderate range may not be detrimental and could even be beneficial for some matrices, but excessive moisture should be avoided. Researchers are advised to systematically optimize these parameters for their specific raw material and target compounds using the structured experimental protocols and workflows outlined in this guide. A methodical approach to preparation ensures not only higher yields but also greater process consistency and economic viability, forming a solid basis for any research or development project in the field of ScCO2 extraction.
Supercritical carbon dioxide (scCOâ‚‚) is a superior solvent for the extraction of non-polar to moderately polar bioactive compounds from natural sources, favored for its tunable physicochemical properties, low toxicity, and environmental friendliness [20] [66]. However, its inherent non-polar nature, with a polarity comparable to that of hexane, presents a significant limitation for pharmaceutical researchers aiming to extract more polar drug compounds such as many alkaloids, flavonoids, and glycosides [67] [20]. This polarity gap can result in low extraction yields and inefficient recovery of target polar molecules, limiting the applicability of scCOâ‚‚ in comprehensive drug development workflows.
The strategic use of polar co-solvents, or modifiers, directly addresses this fundamental challenge. Ethanol, in particular, has emerged as the preferred co-solvent for pharmaceutical applications due to its favorable safety profile, polarity, and miscibility with scCOâ‚‚ [67] [20]. By adding ethanol to the scCOâ‚‚ system, the overall polarity of the supercritical fluid is increased, thereby enhancing its capacity to solubilize a wider range of polar drug compounds. This guide provides an in-depth technical examination of ethanol-modified scCOâ‚‚ extraction, offering detailed methodologies, data analysis, and practical optimization strategies for researchers and scientists in drug development.
Mechanism of Polarity Enhancement with Ethanol
The enhancement of scCOâ‚‚'s solvent power through ethanol addition is not a simple dilution effect but involves complex molecular-level interactions. The primary function of ethanol is to increase the polarity of the supercritical fluid mixture. Carbon dioxide is a linear, non-polar molecule with a zero dipole moment, whereas ethanol possesses a significant dipole moment and the ability to form hydrogen bonds. When introduced into scCOâ‚‚, ethanol molecules effectively reduce the cohesive energy density of the fluid, improving its ability to interact with and solvate polar solute molecules through dipole-dipole interactions and hydrogen bonding [67].
Beyond modifying bulk solvent polarity, ethanol exerts several other critical mechanisms that enhance overall extraction efficiency. Research indicates that ethanol can cause the solid plant matrix to swell, allowing the supercritical fluid to penetrate more deeply into the cellular structure and promoting better mass transfer of solutes into the fluid phase [67]. Furthermore, ethanol may interact directly with the solute-matrix complex, effectively lowering the activation energy required for desorption of target compounds from their binding sites within the plant material [67]. It's crucial to recognize that these effects are concentration-dependent, with an optimal range typically existing beyond which extraction efficiency may plateau or even decrease due to potential phase separation or reduced COâ‚‚-solute interactions [67] [68].
Diagram 1: This flowchart illustrates the primary mechanisms through which ethanol enhances the extraction of polar drug compounds in supercritical COâ‚‚ systems, highlighting the relationship between molecular interactions and improved extraction outcomes.
Quantitative Effects of Ethanol on Extraction Yield and Efficiency
The impact of ethanol modification on scCOâ‚‚ extraction performance has been quantitatively demonstrated across multiple scientific studies involving various plant materials and target compounds. The following table consolidates key experimental findings that highlight the significant enhancement in extraction yield and bioactive compound recovery achievable through ethanol addition.
Table 1: Quantitative Effects of Ethanol Modification on scCOâ‚‚ Extraction Efficiency
| Plant Material | Target Compounds | Optimal Ethanol Concentration | Yield Without EtOH | Yield With EtOH | Enhancement Factor | Reference Conditions |
|---|---|---|---|---|---|---|
| Leptocarpha rivularis Leaves | Total extract (flavonoids, terpenes) | 2 wt.% | 14.7 g/kg[dry basis] | 53.1 g/kg[dry basis] | 3.6x | 60°C, 20 MPa [67] |
| Balanced Cannabis Flower | Cannabinoids (THC/CBD) | 5% w/v | 16.1% w/v (at 818 kg/m³ density) | 18.2% w/v (at 818 kg/m³ density) | 1.13x | 40-55°C, Varying Pressure [68] |
| Hemp Seed | Oil & Phenolic Compounds | 10% | 28.83% | 30.13% | 1.05x | 50°C, 20 MPa, 244 min [48] |
| Sage (Salvia officinalis L.) | Total extract | 1 wt.% | Baseline | 3.6x increase | 3.6x | Not Specified [67] |
The data reveals that ethanol concentration must be carefully optimized for each specific application, as excess modifier can sometimes reduce extraction efficiency. For instance, in the extraction of Cordia verbenacea, the yield decreased when ethanol was used in excess, attributed to reduced COâ‚‚-extract interactions and potential phase separation issues [67]. Similarly, a study on sage reported a decrease in extraction yield when ethanol concentration was increased from 1 wt.% to 2 wt.% [67]. This underscores the importance of methodical optimization for each unique plant matrix and target compound system.
Table 2: Influence of Ethanol on Bioactive Compound Recovery in Hemp Seed Oil [48]
| Bioactive Parameter | scCOâ‚‚ Without Co-solvent | scCOâ‚‚ With 10% Ethanol | Percentage Increase |
|---|---|---|---|
| Total Phenolic Content (GAE mg/kg) | 217.42 | 294.15 | 35.3% |
| Total Tocopherols (mg/kg) | 401.55 | 484.38 | 20.6% |
| Oxidative Stability Index (h) | 22.15 | 28.01 | 26.5% |
| Identified Phenolic Compounds | 18 | 26 | 44.4% |
The enhanced recovery of phenolic compounds and antioxidants demonstrated in Table 2 is particularly relevant for pharmaceutical applications where these bioactive components often contribute to therapeutic efficacy. The identification of 26 distinct phenolic compounds with ethanol modification, compared to only 18 without, highlights the remarkable ability of ethanol to broaden the spectrum of extractable polar metabolites [48].
Experimental Protocols and Methodologies
Standardized Workflow for Ethanol-Modified scCOâ‚‚ Extraction
Implementing a robust experimental protocol is essential for achieving reproducible and efficient extraction of polar drug compounds using ethanol-modified scCOâ‚‚. The following section outlines a comprehensive methodology suitable for research and development activities in pharmaceutical applications.
Diagram 2: Comprehensive experimental workflow for ethanol-modified scCOâ‚‚ extraction, illustrating key procedural steps and critical quality control considerations for each stage of the process.
Detailed Step-by-Step Protocol
Plant Material Preparation
- Begin with authenticated plant material (aerial parts, leaves, roots, or seeds depending on target compounds).
- Dry the plant material at 40-60°C in a controlled oven until moisture content reaches <10% to prevent ice formation and maintain scCO₂ permeability [68].
- Mill or grind the dried material to a consistent particle size of 150-500 μm using an analytical mill. Sieve to ensure homogeneity, as particle size significantly affects mass transfer kinetics [67].
Co-solvent Preparation
- Use high-purity, food-grade ethanol (≥99.9%) to avoid introduction of contaminants that could interfere with subsequent analysis [68].
- For static modification: Pre-mix ethanol with the plant matrix at the desired ratio (typically 2-10% w/w) before loading into the extraction vessel [68].
- For dynamic modification: Utilize a high-pressure co-solvent pump to deliver ethanol at a controlled rate directly into the scCOâ‚‚ stream.
Extraction Vessel Packing
- Accurately weigh the prepared plant material (1-5 g for analytical scale; 100-500 g for pilot scale).
- Pack the extraction vessel uniformly to avoid channeling, which can reduce extraction efficiency. Mix with an inert packing material like glass beads if necessary for small samples.
- For static ethanol modification, ensure even distribution of the ethanol-treated sample throughout the vessel.
System Pressurization and Extraction
- Set the extraction temperature according to optimized parameters (typically 40-60°C for thermolabile compounds).
- Pressurize the system slowly to the target pressure (commonly 20-30 MPa for polar compounds) using a high-pressure pump.
- Maintain scCO₂ density within the optimal range (typically 818-911 kg/m³ for high yields) [68].
- Initiate the dynamic extraction phase with a COâ‚‚ flow rate of 0.25-2 kg/h depending on vessel size [48].
- Continue extraction for a predetermined time (120-300 minutes) or until exhaustive extraction is achieved.
Separation and Collection
- Direct the scCOâ‚‚-extract mixture to a separation vessel maintained at lower pressure (5-10 MPa) and/or higher temperature to decrease solvent power and precipitate the extract.
- For fractional collection, employ multiple separators in series with progressively lower pressures to fractionate compounds based on solubility.
- Collect the extract in amber glass vials and store at -20°C until analysis to preserve compound integrity.
Analytical Methodologies for Extract Characterization
- Chromatographic Analysis: Employ Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) with C18 columns and UV/Photodiode Array detection for quantification of target compounds (e.g., cannabinoids, flavonoids) [68]. Use a binary mobile phase system (e.g., water and methanol with 0.07% phosphoric acid) with gradient elution.
- Bioactivity Screening: Evaluate antimicrobial activity using standard microdilution assays against Gram-positive and Gram-negative bacteria [69]. Assess antioxidant capacity through DPPH radical scavenging, Ferric Reducing Antioxidant Power (FRAP), and β-carotene bleaching assays [67].
- Compound Identification: Utilize HPLC-DAD/ESI-MS² for detailed phenolic profiling and structural characterization of unknown compounds [48].
Optimization Strategies and Experimental Design
Effective optimization of ethanol-modified scCOâ‚‚ extraction requires systematic investigation of critical process parameters and their interactive effects. Response Surface Methodology (RSM) with Box-Behnken or Central Composite Designs has been successfully employed to model complex parameter relationships and identify optimal conditions [67] [48].
Table 3: Key Parameters for Optimizing Ethanol-Modified scCOâ‚‚ Extraction
| Parameter | Experimental Range | Impact on Extraction | Optimization Consideration |
|---|---|---|---|
| Ethanol Concentration | 2-20% (w/w or v/v) | Increases polarity & yield up to optimum; excess may reduce efficiency [67] | Compound-specific optimum typically 5-10%; higher for more polar compounds |
| Pressure | 10-35 MPa | Directly affects scCOâ‚‚ density & solvating power [48] | Higher pressure (20-30 MPa) preferred for higher molecular weight polar compounds |
| Temperature | 40-60°C | Dual effect: increases vapor pressure but decreases density [48] | Balance between compound stability & solubility; typically 50-60°C for polar compounds |
| Extraction Time | 120-300 min | Impacts exhaustive recovery & process economics [48] | Most efficient yield typically achieved in 180-240 min for many plant matrices |
| COâ‚‚ Flow Rate | 0.25-2 kg/h | Affects mass transfer & contact time [48] | Optimize for minimum solvent consumption while maintaining equilibrium |
Statistical optimization using a Box-Behnken design with three factors (pressure, temperature, and ethanol concentration) at three levels each has proven effective for modeling extraction responses. This approach typically requires 14-17 experimental runs and generates second-order polynomial equations that accurately predict yields under various parameter combinations [67] [48]. Analysis of Variance (ANOVA) with F-test and p-value (p ≤ 0.01) calculations validates model significance, while coefficients of determination (R² > 0.94) indicate excellent correlation between predicted and experimental values [48].
Essential Research Reagent Solutions and Equipment
Successful implementation of ethanol-modified scCOâ‚‚ extraction requires access to specific high-quality reagents and specialized equipment. The following table details the essential components of the research toolkit for this methodology.
Table 4: Essential Research Reagent Solutions and Equipment for scCOâ‚‚ Extraction with Ethanol Modification
| Category | Specific Items | Technical Specifications | Function/Purpose |
|---|---|---|---|
| Solvents & Reagents | Carbon Dioxide | 99.99% purity (food-grade) | Primary supercritical fluid solvent [68] |
| Ethanol (co-solvent) | ≥99.9% purity (food-grade) | Polarity modifier for enhanced extraction of polar compounds [68] | |
| HPLC-grade solvents | Methanol, Acetonitrile, Water | Mobile phase preparation for analytical quantification [68] | |
| Reference standards | Target compounds (e.g., berberine, quercetin, cannabinoids) | Quantification and method validation [67] | |
| Extraction Equipment | Supercritical Fluid Extractor | COâ‚‚ pump, co-solvent pump, extraction vessel, pressure regulators, separators | Core extraction system capable of withstanding high pressures [68] |
| Extraction vessels | 100 mL (analytical) to >200 L (industrial) | Contain plant material during extraction [70] | |
| Chiller/Heater units | Precision temperature control (±1°C) | Maintain critical temperature conditions [68] | |
| High-pressure pumps | Precision flow control (±0.1 mL/min) | Deliver CO₂ and co-solvent at consistent rates [68] | |
| Analytical Instruments | HPLC system | With UV/PDA detector, C18 column | Quantification of target bioactive compounds [68] |
| GC-MS system | With appropriate columns and injectors | Volatile compound analysis and identification [71] | |
| Spectrophotometer | UV-Vis capability | Total phenolic, flavonoid, and antioxidant assays [67] |
Ethanol modification represents a powerful strategy for overcoming the inherent polarity limitations of supercritical CO₂ extraction, enabling researchers to effectively recover a broader spectrum of polar drug compounds from plant materials. The strategic addition of typically 2-10% ethanol can enhance extraction yields by 1.1 to 3.6 times while significantly improving the recovery of critical bioactive compounds such as flavonoids, phenolic compounds, and alkaloids [67] [48]. The optimization of process parameters—particularly ethanol concentration, pressure, and temperature—through statistical experimental design is essential for achieving maximum efficiency and reproducibility.
Future advancements in ethanol-modified scCOâ‚‚ extraction will likely focus on several key areas: the development of continuous extraction processes with integrated online analysis, the implementation of AI-driven optimization systems for real-time parameter adjustment, and the exploration of novel co-solvent mixtures for enhanced selectivity [70]. Additionally, the growing emphasis on sustainable pharmaceutical manufacturing will further drive adoption of this green technology, particularly as equipment costs decrease and modular systems become more accessible to research laboratories [72]. As these trends converge, ethanol-modified scCOâ‚‚ extraction is poised to become an increasingly indispensable tool in the drug development pipeline, enabling more efficient and environmentally responsible discovery of therapeutic compounds from natural sources.
Leveraging Machine Learning and RSM for Predictive Modeling and Parameter Optimization
Supercritical carbon dioxide (scCOâ‚‚) extraction has emerged as a pivotal green technology in pharmaceutical and nutraceutical industries, offering significant advantages over conventional organic solvents, including non-toxicity, recyclability, and tunable solvating power through adjustments in temperature and pressure [32] [73]. The efficiency of this technology hinges on precise optimization of extraction parameters and accurate prediction of solute solubility, which directly influences process design, product yield, and final product quality [74]. Traditionally, response surface methodology (RSM) has been the cornerstone of extraction parameter optimization. However, the field is increasingly witnessing a paradigm shift toward machine learning (ML) models, which excel at capturing complex, non-linear relationships inherent in scCOâ‚‚ processes [32] [75]. This technical guide provides an in-depth examination of both RSM and ML frameworks, detailing their application for predictive modeling and parameter optimization within supercritical COâ‚‚ extraction research.
Machine Learning for Predictive Solubility Modeling
Accurate prediction of drug solubility in scCOâ‚‚ is crucial for efficient pharmaceutical process design, including particle engineering and supercritical fluid-based extraction [32] [73]. Machine learning models have demonstrated remarkable capability in this domain, outperforming traditional thermodynamic models and empirical correlations by learning complex, non-linear relationships directly from data without relying on predefined physical equations [32].
Key Algorithms and Performance Metrics
Recent studies have systematically evaluated multiple machine learning algorithms for predicting drug solubility in scCO₂. The performance of these models is typically assessed using statistical metrics such as R-squared (R²), Root Mean Square Error (RMSE), and Average Absolute Relative Deviation (AARD) [32] [76].
Table 1: Performance Comparison of Machine Learning Models for Drug Solubility Prediction in scCOâ‚‚
| Model | R² | RMSE | AARD (%) | Dataset Size | Key Input Features | Reference |
|---|---|---|---|---|---|---|
| XGBoost | 0.9984 | 0.0605 | N/A | 1726 data points (68 drugs) | T, P, Ï, Tc, Pc, ω, MW, Tm | [32] |
| CNN | 0.9839 | N/A | N/A | 744 data points | Tm, MW, P, T | [76] |
| CatBoost | 0.9795 | N/A | N/A | 744 data points | Tm, MW, P, T | [76] |
| AdaBoost-KNN | 0.9945 | N/A | N/A | 45 data points (Letrozole) | T, P | [46] |
| Ensemble (XGBR+LGBR+CATr) | 0.9920 | 0.08878 | N/A | 110 experimental samples | T, P, MW, Tm | [47] |
| Quantile Gradient Boosting | 0.985 | N/A | N/A | 40 data points (Paracetamol) | T, P | [75] |
The Extreme Gradient Boosting (XGBoost) algorithm has demonstrated exceptional performance in solubility prediction, achieving an R² value of 0.9984 and RMSE of 0.0605 on a large dataset comprising 1726 experimental data points across 68 different drugs [32]. Notably, 97.68% of the data points fell within the model's applicability domain, highlighting its strong predictive reliability [32]. Comparative analyses reveal that tree-based ensembles and deep learning approaches consistently outperform linear models, with Convolutional Neural Networks (CNN) and CatBoost also delivering competitive performance [76].
Input Feature Selection and Engineering
Feature selection critically influences model performance. While temperature (T) and pressure (P) are fundamental inputs, incorporating drug-specific properties significantly enhances predictive accuracy [32]. The most impactful features, as identified through SHAP (SHapley Additive exPlanations) analysis, include:
- Molecular Weight (MW): Most influential variable according to feature importance analysis [76]
- Pressure (P): Second most significant parameter [76]
- Temperature (T): Critical for capturing solubility trends [76]
- Melting Point (Tm): Important molecular descriptor [76]
- Additional Thermodynamic Properties: Critical temperature (Tc), critical pressure (Pc), acentric factor (ω), and COâ‚‚ density (Ï) further improve model robustness [32]
Optimization Algorithms for Hyperparameter Tuning
The integration of bio-inspired optimization algorithms with ML models has emerged as a powerful strategy for enhancing predictive accuracy:
- Golden Eagle Optimizer (GEOA): Successfully applied to optimize K-Nearest Neighbors (KNN) models for predicting Letrozole solubility, achieving R² scores of 0.9907 for KNN, 0.9945 for AdaBoost-KNN, and 0.9938 for Bagging-KNN [46]
- Whale Optimization Algorithm (WOA): Effectively employed for hyperparameter tuning of ensemble models including Extra Trees (ETR), Random Forest (RFR), Gradient Boosting (GBR), and Quantile Gradient Boosting (QGB) [75]
- Hippopotamus Optimization Algorithm (HOA): Utilized to enhance ensemble models combining XGBoost, LightGBM, and CatBoost, achieving an R² of 0.9920 [47]
Figure 1: Machine Learning Workflow for scCOâ‚‚ Solubility Prediction. The diagram illustrates the systematic process from data collection to model deployment, highlighting critical stages including feature selection and hyperparameter optimization.
Response Surface Methodology for Parameter Optimization
Response Surface Methodology (RSM) remains a widely adopted statistical technique for optimizing complex processes by modeling the relationship between input variables and response outcomes [74]. In scCOâ‚‚ extraction, RSM enables researchers to efficiently identify optimal parameter combinations while minimizing experimental runs.
Fundamental Principles and Experimental Design
RSM employs mathematical and statistical techniques to analyze empirical models and optimize processes [77]. The methodology typically involves:
- Identifying Critical Process Parameters: Temperature, pressure, and extraction time are most frequently optimized [77]
- Selecting Appropriate Experimental Design: Central Composite Design (CCD) is commonly employed for scCOâ‚‚ extraction studies [78]
- Model Fitting and Statistical Analysis: Developing quadratic polynomial equations to describe factor-response relationships
- Response Optimization: Identifying parameter combinations that maximize or minimize desired outcomes
Application Case Study: Currant Pomace Extraction
A recent study demonstrated the application of RSM for optimizing scCOâ‚‚ extraction from blackcurrant and redcurrant pomace [77]. The research investigated the effects of pressure, temperature, and time on the recovery of fat, protein, and total phenolic compounds (TPCs).
Table 2: Optimal scCOâ‚‚ Extraction Parameters for Different Bioactive Compounds from Currant Pomace
| Target Compound | Pressure (bar) | Temperature (°C) | Time (min) | Drying Method | Yield/Content | Reference |
|---|---|---|---|---|---|---|
| Protein | 400 | 30 | 60 | Freeze-drying | 14.5% | [77] |
| Total Phenolic Compounds (TPCs) | 500 | 40 | 60 | Freeze-drying | 24.60 mg GAE/g d.w. | [77] |
| Fat | 300-500 | 30-50 | Varying | Conventional & Freeze-drying | Varying yields | [77] |
The study identified pressure and time as the most influential process variables, enhancing solvent density and mass transfer during extraction [77]. Freeze-drying pretreatment consistently yielded superior results compared to conventional hot-air drying, preserving thermolabile bioactive compounds despite higher energy requirements [77].
Advanced RSM Applications: Combined Extraction Techniques
Recent research has explored the optimization of combined subcritical water and scCO₂ extraction for enhanced phenolics recovery from coffee byproducts [78]. Using Design Expert V.13 with Central Composite Design (CCD), parameters including extraction time (30-60 min), temperature (180-220°C), and solid-to-water ratio (0.024-0.027 g/mL) were systematically analyzed.
For spent coffee grounds (SCG), optimal conditions were determined as 198°C, 0.027 g/mL solid-to-water ratio, and 60 minutes, yielding a total phenolic content (TPC) of 217.26 mg GAE/g DW [78]. Coffee cherry pulp (CCP) exhibited even higher potential under optimized conditions of 189°C, 0.024 g/mL solid-to-water ratio, and 54 minutes, resulting in a TPC of 230.13 mg GAE/g DW [78].
Figure 2: RSM Optimization Workflow for scCOâ‚‚ Extraction. The diagram outlines the systematic approach for optimizing supercritical COâ‚‚ extraction parameters, highlighting critical stages of experimental design and statistical analysis.
Comparative Analysis and Hybrid Approaches
Both ML and RSM offer distinct advantages for scCOâ‚‚ process optimization, with their relative effectiveness depending on specific research objectives, data availability, and computational resources.
Performance and Applicability Considerations
- Data Requirements: ML models typically require larger datasets (hundreds to thousands of data points) for robust training [32], while RSM can generate effective models with fewer, strategically designed experiments [77]
- Computational Complexity: Advanced ML implementations involve sophisticated optimization algorithms and hyperparameter tuning [46], whereas RSM relies on established statistical packages with lower computational demands
- Interpretability: RSM provides explicit mathematical relationships between factors and responses [77], while ML models often function as "black boxes," requiring techniques like SHAP analysis for interpretability [76]
- Generalization Capability: Properly trained ML models can extrapolate beyond their training range more effectively than RSM models, which are typically constrained to the experimental design space [32]
Emerging Hybrid Frameworks
Recent research trends indicate a growing interest in hybrid approaches that leverage the strengths of both methodologies:
- RSM-Informed Feature Selection: Using RSM to identify critical parameters before ML modeling
- Ensemble ML-RSM Prediction: Combining predictions from both approaches for enhanced reliability
- Sequential Optimization: Employing RSM for preliminary optimization followed by ML for fine-tuning and robustness analysis
Experimental Protocols and Methodologies
Standardized Experimental Protocol for scCOâ‚‚ Solubility Measurement
Based on analysis of multiple studies [32] [46], a comprehensive experimental protocol for determining drug solubility in scCOâ‚‚ includes:
Sample Preparation:
- Pre-dry drug compounds to remove moisture
- Characterize initial particle size and morphology
- Determine key physicochemical properties (MW, Tm, etc.)
Extraction Process:
- Load known quantity of drug into high-pressure vessel
- Pressurize system with COâ‚‚ to target pressure (typically 12-35 MPa)
- Maintain temperature (308-338 K) using precision circulator
- Equilibrate system with continuous stirring for specified duration
Sampling and Analysis:
- Slowly depressurize saturated scCOâ‚‚ through restrictor valve
- Collect dissolved solute in appropriate solvent trap
- Quantify drug concentration using validated analytical methods (HPLC, UV-Vis)
- Calculate mole fraction solubility based on collected mass and COâ‚‚ volume
Machine Learning Implementation Protocol
For researchers implementing ML models for solubility prediction, the following standardized protocol is recommended [32] [46]:
Data Preprocessing:
- Normalize features using Min-Max scaling or standardization
- Detect and handle outliers using Isolation Forest algorithm
- Split dataset into training (80%) and testing (20%) sets
Model Development:
- Select appropriate algorithm based on dataset size and complexity
- Implement hyperparameter optimization using bio-inspired algorithms
- Train model with k-fold cross-validation (typically k=10)
Model Validation:
- Evaluate performance on unseen test data
- Analyze residuals and applicability domain using William's plot
- Conduct sensitivity analysis using SHAP or similar frameworks
Table 3: Essential Research Reagent Solutions and Materials for scCOâ‚‚ Extraction Studies
| Category | Specific Items | Technical Specifications | Function/Purpose |
|---|---|---|---|
| Solvents & Reagents | Carbon Dioxide (CO₂) | High purity (≥99.9%) | Primary supercritical solvent |
| HPLC-grade solvents | Methanol, Ethanol, Acetonitrile | Sample collection and analysis | |
| Analytical standards | Certified reference materials | Quantification and calibration | |
| Equipment | Supercritical fluid extractor | High-pressure (≥500 bar), temperature-controlled | Core extraction system |
| Analytical balances | Precision ±0.0001 g | Accurate mass measurement | |
| HPLC system | With UV/RI detectors | Solute quantification | |
| Freeze dryer | Capable of -40°C, 0.001 mbar | Sample preservation | |
| Software | Python/R | With scikit-learn, TensorFlow | Machine learning implementation |
| Design Expert | Version 13 or higher | RSM experimental design | |
| Molecular modeling | RDKit, alvaDesc | Molecular descriptor calculation |
The integration of machine learning and response surface methodology represents a powerful framework for advancing supercritical COâ‚‚ extraction research. While RSM remains valuable for designing efficient experiments and understanding parameter interactions within defined spaces, ML approaches offer superior predictive accuracy and generalization capability for complex solubility relationships. The choice between these methodologies should be guided by specific research objectives, available data, and required interpretability.
Future directions in this field will likely focus on hybrid approaches that leverage the experimental efficiency of RSM with the predictive power of ML, ultimately accelerating the development of sustainable pharmaceutical processes using supercritical carbon dioxide technology. As both methodologies continue to evolve, their synergistic application will play an increasingly important role in optimizing green extraction processes across pharmaceutical, nutraceutical, and food industries.
Validating SFE-CO2: Comparative Analysis and Industry Outlook
The selection of an extraction method is a critical determinant in the yield, purity, and biological activity of natural product extracts for pharmaceutical, nutraceutical, and cosmetic applications. This technical guide provides a comparative analysis of two prominent techniques: conventional hexane extraction and supercritical fluid extraction using carbon dioxide (SFE-CO2). While hexane extraction has been the industrial mainstay due to its high efficiency, SFE-CO2 emerges as a superior alternative that eliminates toxic solvent residues, enhances the recovery of sensitive bioactives, and aligns with green chemistry principles. This whitepaper synthesizes current research to demonstrate that SFE-CO2 offers significant advantages in producing high-purity, pharmaceutically relevant extracts, despite its higher initial investment and technical complexity. The following sections provide a detailed examination of quantitative performance data, safety profiles, experimental protocols, and implementation considerations to inform researchers and drug development professionals.
The efficacy of a bioactive compound is intrinsically linked to the method used for its isolation from the raw biomass. The choice of extraction technique profoundly influences the final product's chemical profile, yield, stability, and therapeutic potential [79]. Conventional solvent-based methods, particularly hexane extraction, have dominated industrial-scale operations for decades due to their high efficiency and low cost. However, growing regulatory pressures and a consumer shift towards "clean-label" products have intensified the search for safer, more sustainable alternatives [80] [72].
Supercritical carbon dioxide (SC-CO2) extraction represents a technologically advanced, solvent-free method. Carbon dioxide, when heated and pressurized beyond its critical point (31.1°C and 7.29 MPa), assumes a supercritical state with unique properties: liquid-like density, gas-like diffusivity and viscosity, and zero surface tension [81]. This allows SC-CO2 to penetrate porous solid matrices efficiently and dissolve lipophilic compounds. The solvent power of SC-CO2 is highly tunable; slight adjustments to pressure and temperature can selectively target different compound classes [48] [82]. Upon depressurization, CO2 reverts to a gas, leaving behind a pure, solvent-free extract and eliminating the need for costly and potentially degrading purification steps [83]. This technical guide delves into a point-by-point comparison of these two methodologies, providing a scientific foundation for their evaluation within a research context.
Comparative Performance Analysis
Extraction Yield and Efficiency
Extraction yield is a primary metric for evaluating process efficiency. The performance varies significantly based on the plant material and operational parameters.
Hexane Extraction is renowned for its high efficiency, achieving oil recovery rates of up to 99% from oil-rich seeds due to its excellent solvation power for non-polar compounds [80]. For instance, poppy seeds yielded 29.0% oil with hexane, while pumpkin seeds yielded 24.6% [80]. However, this method's selectivity is low, often co-extracting unwanted compounds like chlorophyll, which can impart undesirable colors and odors to the crude extract, necessitating further refining [80] [83].
SFE-CO2 yield is highly dependent on process parameters. It has proven particularly effective for seeds with lower oil content or small seed size, such as linden, marigold, poppy, and flaxseed [80]. For example, SFE-CO2 achieved a 26.8% yield from flaxseed, compared to ~10.7% with hexane [80]. Pressure is a critical factor; increasing pressure enhances CO2 density and solvation power, thereby increasing yield [48]. The "crossover phenomenon" is observed where the relationship between temperature and yield changes above a certain crossover pressure, allowing for optimized parameter selection [84].
Table 1: Comparative Oil Yields from Various Plant Materials Using Different Extraction Methods
| Plant Material | Hexane Extraction Yield (%) | SFE-CO2 Yield (%) | Cold Pressing Yield (%) |
|---|---|---|---|
| Pumpkin Seed | 24.6 | 18.8 | 10.7 |
| Flaxseed | ~10.7 | 26.8 | - |
| Linden Seed | 10.8 | 16.3 | 9.7 |
| Poppy Seed | 29.0 | 28.3 | - |
| Apricot Seed | - | - | 25.0 (viable alternative) |
Table 2: Impact of SFE-CO2 Parameters on Hemp Seed Oil Yield and Bioactives [48]
| Parameter | Effect on Oil Yield | Effect on Bioactive Compounds (e.g., Phenolics) | Key Influence |
|---|---|---|---|
| Pressure | Positive linear effect; increased pressure increases yield. | Higher pressure can enhance recovery. | Primary factor for yield. |
| Temperature | Complex effect; can be positive or negative depending on pressure (crossover phenomenon). | Higher temperatures may degrade heat-sensitive compounds. | Impacts compound stability. |
| Co-solvent (Ethanol) | Modest increase in yield (e.g., from 28.83% to 30.13% for hemp seed oil). | Significant enhancement; increases total phenolic content and tocopherols. | Critical for polar compound extraction. |
Purity and Bioactive Compound Profile
The purity and compositional integrity of the extract are paramount for pharmaceutical applications.
Hexane Extraction is effective for recovering non-polar triglycerides and fatty acids. Studies on blackberry pomace and various seed oils show that the fatty acid profile from hexane extraction is largely comparable to that of SFE-CO2 [80] [83]. However, the prolonged heating required for solvent removal can degrade heat-sensitive bioactive compounds such as antioxidants, phytosterols, and carotenoids [80] [79]. Furthermore, the crude extract contains solvent residues that must be removed through additional refining steps, which can further degrade the quality of the oil [80] [83].
SFE-CO2 excels in preserving sensitive bioactives due to its operation in an oxygen-free, light-free environment and typically lower processing temperatures [81]. It demonstrates superior selectivity for valuable unsaponifiable matter:
- Phytosterols: SFE-CO2 significantly improves the total phytosterol content in oils. Pumpkin seed oil, for instance, showed a marked increase in phytosterols when extracted with SFE-CO2 [80].
- Carotenoids: Research on Rhodotorula toruloides yeast found that SFE-CO2 better preserved carotenoids like torularhodin and torulene, which degraded during the saponification step required in conventional acetone extraction. The total carotenoid concentration was dramatically higher with SFE-CO2 (332.09 µg/g) compared to acetone extraction (19.9 µg/g) [81].
- Fatty Acids: SFE-CO2 extracts often have a higher proportion of unsaturated fatty acids. In yeast lipid extraction, the SC-CO2 method resulted in a greater share of unsaturated lipids compared to the conventional Folch method [81].
- Phenolic Compounds: The addition of a polar co-solvent like ethanol (5-10%) to SC-CO2 can significantly boost the yield of polar antioxidants. In hemp seed oil, 10% ethanol increased the total phenolic content and allowed for the identification of 26 distinct phenolic compounds, including N-trans-caffeoyltyramine and cannabisins [48].
Safety and Environmental Impact
The safety profile of an extraction method encompasses operator safety, consumer safety, and environmental sustainability.
Hexane Extraction poses significant concerns. Hexane is classified as a hazardous air pollutant and is identified as toxic to reproduction by the European Chemical Agency (ECHA) [80]. It is hepatotoxic in rodents, and chronic exposure in humans can lead to polyneuropathy [80]. From an environmental perspective, hexane is an ozone precursor, and its industrial use leads to emissions that contribute to air pollution [80]. Residual solvent in the final product, albeit in trace amounts, is a persistent concern for consumer safety, especially in pharmaceuticals and nutraceuticals [80] [72].
SFE-CO2 is markedly safer. CO2 is non-toxic, non-flammable, and readily available [80]. It is granted Generally Recognized As Safe (GRAS) status by the US FDA, allowing for unrestricted use in food and pharmaceutical extracts [80] [48]. The process leaves no solvent residues in the final product, ensuring ultimate consumer safety [48]. Environmentally, CO2 used in SFE can be recycled within the system, and the process has a minimal environmental footprint compared to volatile organic solvents [80] [72]. This aligns with the principles of green chemistry and meets the growing demand for clean-label products, driving its adoption in sensitive industries [72].
Table 3: Safety and Environmental Impact Comparison
| Factor | Hexane Extraction | SFE-CO2 Extraction |
|---|---|---|
| Solvent Toxicity | Hepatotoxic; causes polyneuropathy; toxic to reproduction. | Non-toxic, non-flammable, GRAS status. |
| Residual Solvent | Present in crude extract, requires removal; risk of trace residues. | None; CO2 evaporates completely. |
| Environmental Impact | Ozone precursor; hazardous air pollutant; volatile organic compound (VOC). | Minimal; CO2 can be recycled; often uses waste CO2. |
| Regulatory Status | Classified as hazardous; use is restricted and monitored. | Encouraged as a green and sustainable technology. |
Experimental Protocols
Standard Protocol for SFE-CO2 Extraction
The following protocol for extracting hemp seed oil, optimized using Response Surface Methodology (RSM), serves as a robust template for similar materials [48].
- Sample Preparation: Seeds are crushed and sieved to a uniform particle size (e.g., 500 μm). The moisture content should be controlled, as high moisture can impede CO2 diffusion.
- Equipment Setup: Pack the extraction vessel (e.g., 1L capacity) with the prepared biomass. Ensure the system is leak-free. The typical setup includes a CO2 cylinder, a chiller, a high-pressure pump, a co-solvent pump (if used), an extraction vessel housed in a temperature-controlled oven, and a separator for collecting the extract.
- Parameter Optimization (Based on RSM):
- Temperature: Set based on the target compound's thermal stability. A common range is 40-60°C. For hemp seed oil, 50°C was optimal [48].
- Pressure: Optimize for maximum yield and bioactive recovery. For hemp seed oil, 20 MPa was found to be optimal. Higher pressures generally increase yield but also operational costs [48].
- CO2 Flow Rate: Maintain a constant flow rate (e.g., 0.25 kg/h for small-scale, 25 g/min for larger scale) [48] [82].
- Extraction Time: Determine dynamically; for hemp seed oil, 244 minutes was sufficient under optimized conditions [48].
- Co-solvent: For polar bioactives, use a polar modifier like ethanol. A proportion of 5-10% is often effective. For hemp seed oil, 10% ethanol significantly boosted phenolic content without altering the fatty acid profile [48].
- Extraction and Collection: Initiate the dynamic extraction. The extract, dissolved in SC-CO2, is passed to a separator where pressure is reduced to ambient, causing CO2 to gasify and the extract to precipitate. The extract is collected in a dark vessel and stored at -20°C until analysis.
Standard Protocol for Hexane Extraction (Soxhlet)
This conventional method is widely used as a reference for determining total extractable material [80] [83].
- Sample Preparation: Dry and grind the plant material to a fine powder to increase the surface area for solvent contact.
- Equipment Setup: Load the powdered material into a cellulose thimble and place it in the main chamber of the Soxhlet apparatus. Fill the boiling flask with a sufficient volume of n-hexane.
- Extraction: Heat the flask to reflux. The hexane vapor condenses and drips onto the sample, extracting the lipids. When the siphon arm fills, it empties the solvent back into the boiling flask. This cycle repeats for a set period (typically 6-8 hours) [82].
- Solvent Removal: After extraction, the hexane in the boiling flask is concentrated using a rotary evaporator under reduced pressure to recover the crude oil. Prolonged heating during this step should be minimized to prevent thermal degradation.
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Materials and Reagents for SFE-CO2 and Hexane Extraction Research
| Item | Function/Description | Research Application Note |
|---|---|---|
| Supercritical CO2 Extractor | System comprising CO2 pump, extraction vessel, pressure/temperature controls, and separator. | Essential for SFE. Systems range from small-scale (<50L) for R&D to large-scale (>200L) for pilot production [72]. |
| Soxhlet Apparatus | Conventional extraction setup for continuous solvent reflux and extraction. | Standard reference method for determining total extractable yield with hexane [83]. |
| Food-Grade Carbon Dioxide | Primary solvent for SFE; must be high purity. | GRAS-status solvent. Its solvent power is tunable via pressure and temperature [80] [48]. |
| n-Hexane | Non-polar organic solvent for conventional lipid extraction. | Highly efficient but hazardous. Requires fume hood and careful handling due to toxicity and flammability [80]. |
| Anhydrous Ethanol | Polar co-solvent for SFE-CO2. | Added to SC-CO2 (typically 5-15%) to significantly improve the extraction yield of polar bioactive compounds like phenolics and flavonoids [48] [85]. |
| Analytical Standards | Pure reference compounds (e.g., phytosterols, tocopherols, specific phenolic compounds). | Critical for calibrating instruments (HPLC, GC) and quantifying specific bioactive compounds in the extracts [80] [48]. |
Implementation and Market Considerations
The transition from conventional to supercritical extraction involves significant strategic considerations. The global SFE extractor market is experiencing significant growth, driven by demand for clean-label products in the pharmaceutical, food, and cosmetic industries [72]. North America and Europe are currently dominant markets, while the Asia-Pacific region is projected to grow the fastest [72].
The primary barrier to adoption is the high initial investment required for SFE equipment and the technical expertise needed for operation and maintenance [72]. This can be prohibitive for small and medium-sized enterprises. In contrast, hexane extraction systems have lower upfront costs and are less complex to operate, though they incur ongoing costs for solvent purchase, waste disposal, and compliance with environmental and safety regulations [80] [72].
However, the market trend clearly favors SFE-CO2. The acquisition of specialized firms by larger corporations and strategic alliances between equipment manufacturers and pharmaceutical companies indicate a consolidation and maturation of the SFE technology market [72]. Furthermore, the imposition of stringent regulatory standards for solvent residues in final products is a key driver pushing industries toward solvent-free alternatives like SFE-CO2 [72].
The comparative analysis unequivocally demonstrates that SFE-CO2 extraction holds a distinct advantage over hexane extraction for applications where purity, solvent residue, and the integrity of sensitive bioactive compounds are critical. While hexane remains a cost-effective choice for high-volume extraction of non-polar oils where ultimate purity is not the primary concern, its associated health and environmental liabilities are significant. SFE-CO2, though requiring a higher initial investment and technical expertise, provides a sustainable, safe, and selective alternative that is increasingly aligned with regulatory trends and consumer preferences. Continued optimization of SFE parameters and the strategic use of co-solvents have the potential to further revolutionize the production of plant-based extracts, solidifying its role as the future of green extraction technology in advanced research and drug development.
The selection of an optimal extraction method is a critical determinant in the quality, bioactivity, and commercial viability of plant-derived oils. Supercritical fluid extraction using carbon dioxide (SFE-CO2) and cold pressing represent two prominent techniques with distinct technological and philosophical approaches. This in-depth technical guide provides a comparative assessment of these methods, focusing on the critical parameters of oil yield, phytosterol content, and antioxidant activity, framed within ongoing research into the fundamentals of SFE-CO2 technology. For researchers and drug development professionals, understanding these distinctions is essential for designing extraction processes that maximize the recovery of valuable bioactive compounds for nutraceutical, cosmetic, and pharmaceutical applications.
The following table summarizes the core performance differences between SFE-CO2 and Cold Pressing extraction methods across key metrics, as established by current research.
Table 1: Core Performance Comparison of SFE-CO2 vs. Cold Pressing
| Performance Metric | Supercritical CO2 (SFE-CO2) | Cold Pressing |
|---|---|---|
| Typical Oil Yield | Superior for most seeds, especially low-oil-content types (e.g., linden, marigold) [80] | Suboptimal; yields range from 10-25% for many seeds [80] |
| Phytosterol Content | Enhances total phytosterol content; significantly higher in oils like pumpkin seed [80] [86] | Lower phytosterol content compared to SFE-CO2 [80] |
| Antioxidant Activity | Yields unsaponifiable matter with high, variable antioxidant potential [80] | Generally lower antioxidant activity in the unsaponifiable fraction [80] |
| Fatty Acid Profile | No significant alteration; comparable to other methods [80] [87] | No significant alteration; comparable to other methods [80] |
| Process Selectivity | Tunable selectivity for compounds like free fatty acids and bioactives; can be enhanced with co-solvents [48] [87] | Low selectivity; extract composition is largely fixed by the raw material [88] |
| Operational Safety | High; uses non-toxic, non-flammable COâ‚‚ in an oxygen-free environment [81] [82] | High; physical process without chemical solvents [80] |
| Environmental Impact | Favorable; COâ‚‚ is recycled, no organic solvent residues [80] [87] | Favorable; no chemical solvents used [80] |
Detailed Quantitative Data Analysis
Oil Extraction Yield
Oil yield is a primary economic and efficiency indicator. A comprehensive 2024 study analyzing six plant species provides direct comparative data, as shown in Table 2 [80] [86].
Table 2: Comparative Oil Yields (%) by Extraction Method and Plant Species [80]
| Plant Seed | SFE-CO2 Yield (%) | Cold Pressing Yield (%) | Hexane Extraction Yield (%) |
|---|---|---|---|
| Pumpkin | 18.8 | 10.7 | 24.6 |
| Flaxseed | 26.8 | - | 10.2 |
| Linden | 16.3 | 9.7 | 10.8 |
| Poppy | Data Incomplete | Data Incomplete | 29.0 |
| Apricot | Data Incomplete | Data Incomplete | Data Incomplete |
| Marigold | Data Incomplete | Data Incomplete | Data Incomplete |
The data indicates that SFE-CO2 is the preferred extraction method for four out of six plant materials, particularly for seeds with lower oil content like linden and marigold [80]. For instance, SFE-CO2 yielded 16.3% from linden seeds compared to 9.7% from cold pressing. However, for some high-oil-content seeds like apricot, cold pressing remains a viable alternative from a yield perspective [80].
Further research on baobab seed oil corroborates this trend, where optimized neat SFE-CO2 yielded 9.3 wt%, which was lower than the 37 wt% achieved by conventional warm pressing [87]. This highlights that while SFE-CO2 is generally superior, the optimal choice can be species-dependent and must be determined empirically.
Phytosterol and Bioactive Content
The unsaponifiable fraction of plant oils, which includes phytosterols, tocopherols, and squalene, is crucial for their functional and nutritional properties. SFE-CO2 demonstrates a marked advantage in enriching this fraction.
- Enhanced Phytosterol Recovery: SFE-CO2 extraction improved the total phytosterol content of the tested oils, with a particularly notable effect on pumpkin seed oil [80] [86]. Phytosterols are known for their cholesterol-lowering and anti-inflammatory activities [80].
- Co-solvent Enhancement: The extractability of polar bioactive compounds like phenolics can be significantly boosted by using ethanol as a co-solvent. In hemp seed oil, SFE-CO2 modified with 10% ethanol increased the total phenolic content (TPC) to 294.15 GAE mg/kg and total tocopherols to 484.38 mg/kg, levels not achievable with neat COâ‚‚ or cold pressing [48].
- Fatty Acid Profile: It is noteworthy that neither SFE-CO2 nor cold pressing significantly alters the fundamental fatty acid composition of the oil [80] [87]. The primary differences lie in the recovery of the minor, but biologically critical, unsaponifiable components.
Antioxidant Activity of Extracts
The antioxidant potential of plant oils is a key indicator of their stability and health-promoting value. Research indicates that the extraction method significantly influences this property.
- Antioxidant Potential: A high variability in the antioxidant potential of the unsaponifiable matter was determined, with pumpkin seed oil showing the highest antioxidant activity among the six species studied [80].
- Correlation with Compounds: Correlation analysis revealed that the antioxidant activity was statistically significantly correlated with specific compounds in the unsaponifiable fraction, including squalene, cycloartenol, and an unidentified compound [80]. This suggests SFE-CO2 may selectively co-extract these antioxidant agents more effectively.
- Comparison with Other Methods: A 2023 study on pumpkin seed oil found that oil from microwave-pretreated pressing (MP) showed superior antioxidant properties in DPPH, FRAP, and ABTS assays compared to both SFE-CO2 and conventional cold pressing (CP) [88]. This indicates that while SFE-CO2 is excellent, hybrid or pre-treatment methods can also be highly effective for maximizing antioxidant capacity.
Experimental Protocols and Methodologies
To ensure reproducibility and provide a clear technical roadmap, this section outlines standard experimental protocols for the comparative assessment of extraction methods.
Sample Preparation Protocol
- Raw Material Sourcing: Acquire plant seeds (e.g., pumpkin, flax, linden) from a reputable supplier, noting the harvest date and storage conditions [80].
- Drying: Dry seeds in an oven at 50°C for 24 hours to reduce moisture content, which can interfere with extraction efficiency [87].
- Comminution: Grind the dried seeds into a fine powder using a laboratory mill (e.g., Retsch Mill MM 200) [87]. The particle size should be standardized, often to ~500 μm, by sieving [48].
- Storage: Store the powdered samples in sealed, light-proof containers at -20°C until extraction to prevent oxidative degradation [81].
SFE-CO2 Extraction Protocol
- System Setup: Utilize a commercial SFE system (e.g., Waters MV-10, Thar Designs SFE-1000) [87] [82]. Ensure all lines and the collection vessel are clean.
- Vessel Packing: Weigh a precise mass (e.g., 2-150 g) of the prepared seed powder and pack it into the extraction vessel [48] [88].
- Parameter Setting: Set the operational parameters. Standard conditions for seed oils are often in the range of 50-60°C and 20-35 MPa [48] [88]. A CO₂ flow rate of 0.25-25 g/min is typical [48] [82].
- Dynamic Extraction: Initiate the dynamic extraction by pumping COâ‚‚ through the vessel for a set duration (e.g., 1-4 hours) [48] [82].
- Extract Collection: Depressurize the fluid to atmospheric pressure in the separator, causing the oil to precipitate. Collect the oil gravimetrically [87].
- Co-solvent Modification (Optional): For enhanced phenolic compound recovery, introduce a polar co-solvent like ethanol (2.5-10%) via a secondary HPLC pump to mix with the COâ‚‚ stream [48] [82].
Cold Pressing Extraction Protocol
- Press Setup: Use a single-screw oil press (e.g., Miramar M222/20F) [88].
- Feeding and Pressing: Feed the whole or lightly crushed seeds into the press hopper. The mechanical screw forces the seeds through a barrel, generating pressure and temperature.
- Temperature Monitoring: Monitor the temperature of the expressed oil, which should ideally remain below 50°C to qualify as a true "cold-pressed" oil [88].
- Oil Collection: Collect the crude oil exiting the press. Filter if necessary to remove large particulate matter [80].
- Post-processing: Store the oil in amber glass vessels under an inert atmosphere (e.g., Nâ‚‚) to preserve its quality [80].
Analytical Methods for Comparative Assessment
- Oil Yield Calculation: Determine yield gravimetrically as (mass of extracted oil / mass of seeds) × 100 [88].
- Phytosterol Analysis: Derivatize the unsaponifiable matter (e.g., with BSTFA + TMCS) and analyze via Gas Chromatography-Mass Spectrometry (GC-MS) [80] [88].
- Antioxidant Activity:
- Fatty Acid Profile: Convert oils to Fatty Acid Methyl Esters (FAMEs) and analyze by Gas Chromatography with a Flame Ionization Detector (GC-FID) [81] [87].
Diagram 1: Experimental workflow for oil extraction and analysis.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key reagents, materials, and equipment essential for conducting the described experiments.
Table 3: Essential Research Reagents and Materials for Extraction and Analysis
| Item Name | Specification / Example | Primary Function in Research |
|---|---|---|
| Supercritical CO2 Extractor | Thar Designs SFE-1000; Waters MV-10 [87] [82] | Core apparatus for performing SFE-CO2 and co-solvent-modified extractions. |
| Single-Screw Oil Press | Miramar M222/20F [88] | Equipment for producing cold-pressed oils under controlled temperature. |
| Carbon Dioxide (CO₂) | Ultrapure (≥99.999%) [87] | The supercritical solvent for SFE; high purity is critical to prevent contamination. |
| Absolute Ethanol | 99.7% purity, GC derivatisation grade [48] [87] | Polar co-solvent for SFE to enhance extraction of phenolic compounds. |
| GC-MS System | Standard System (e.g., Agilent, Thermo) [80] | Identification and quantification of phytosterols and other unsaponifiable compounds. |
| GC-FID System | Standard System [81] [87] | Analysis of fatty acid methyl esters (FAMEs) to determine fatty acid profile. |
| Phytosterol Standards | Campesterol, Stigmasterol, β-Sitosterol [88] | Calibration and quantification of individual phytosterols in the extracted oil. |
| Antioxidant Assay Kits | DPPH, FRAP, ABTS [88] | Standardized reagents for in vitro evaluation of antioxidant capacity. |
| Derivatization Reagent | Bis(trimethylsilyl)trifluoroacetamide (BSTFA) + TMCS [88] | Silanizing agent for derivatizing phytosterols to make them volatile for GC-MS. |
| FAME Mix Standard | Supelco 37-component FAME mix [87] | Reference standard for identifying and quantifying fatty acids via GC-FID. |
The comparative analysis between SFE-CO2 and cold pressing reveals a clear, application-dependent rationale for method selection. SFE-CO2 is a technologically advanced, tunable, and highly efficient method that consistently delivers superior oil yields from diverse feedstocks and excels at enriching oils with valuable phytosterols and antioxidant compounds, especially when modified with co-solvents like ethanol. This makes it the preferred choice for producing high-potency extracts for nutraceutical, pharmaceutical, and high-end cosmetic applications where bioactive content is paramount.
Conversely, cold pressing remains a simple, cost-effective, and solvent-free technique suitable for producing high-quality, "natural" food-grade oils, particularly from seeds with high native oil content. Its limitations in yield and bioactive recovery are offset by its simplicity and consumer appeal.
Future research in SFE-CO2 fundamentals will likely focus on optimizing parameters for novel plant matrices, integrating AI for real-time process control [89], and developing sophisticated multi-stage biorefinery approaches to valorize entire biomass streams [82]. For scientists and drug developers, the choice is not merely between two methods, but between two paradigms: one of maximal bioactive recovery and process control (SFE-CO2), and another of minimal processing and marketing appeal (Cold Pressing).
Evaluating Environmental Impact and Regulatory Compliance
Supercritical carbon dioxide (SC-CO₂) extraction represents a technologically advanced and environmentally sustainable separation method that utilizes carbon dioxide above its critical point (31.1°C and 73.8 bar) as a solvent [20]. In this state, CO₂ possesses unique properties that combine liquid-like density with gas-like diffusivity and viscosity, enabling efficient penetration of biological matrices and extraction of target compounds [16]. The process has gained significant traction across pharmaceuticals, food, cosmetics, and nutraceutical industries as a green alternative to conventional organic solvent-based extraction methods [20] [90]. This technical evaluation examines the environmental advantages and regulatory compliance aspects of SC-CO₂ extraction technology, providing researchers and drug development professionals with comprehensive guidance for implementing this sustainable technology.
The fundamental principle of SC-COâ‚‚ extraction relies on the tunable solvating power of carbon dioxide in its supercritical state. By manipulating temperature and pressure parameters, operators can selectively extract specific compound classes from complex biological matrices [20]. The process is typically performed in a closed-loop system where COâ‚‚ is recycled, minimizing waste and environmental release [91]. Following extraction, the reduction of pressure causes the COâ‚‚ to revert to its gaseous state, leaving behind the extracted compounds without solvent residue [20]. This intrinsic characteristic forms the basis for both the environmental benefits and regulatory advantages of the technology.
Environmental Impact Assessment
Comparative Environmental Advantages
SC-COâ‚‚ extraction offers substantial environmental benefits compared to traditional solvent-based extraction methods, positioning it as a cornerstone technology for sustainable industrial processing. The most significant advantage lies in the complete elimination of hazardous organic solvents, which represent a major environmental concern throughout their lifecycle from production to disposal [20]. Conventional methods using solvents such as n-hexane, chloroform, or methanol generate substantial waste streams requiring specialized treatment and pose risks of atmospheric emissions and groundwater contamination [91] [16]. In contrast, SC-COâ‚‚ systems utilize carbon dioxide, which is non-toxic, non-flammable, and chemically inert [20].
The environmental profile of SC-COâ‚‚ extraction is further enhanced through reduced energy consumption and minimal waste generation. The table below quantifies key environmental advantages compared to traditional solvent extraction methods:
Table 1: Environmental Impact Comparison: SC-COâ‚‚ vs. Traditional Extraction Methods
| Parameter | SC-COâ‚‚ Extraction | Traditional Solvent Extraction | Environmental Significance |
|---|---|---|---|
| Solvent Toxicity | Non-toxic, no chemical residues [20] | Toxic solvents (n-hexane, chloroform) requiring special handling [91] [16] | Eliminates hazardous waste streams, protects water quality |
| Solvent Recovery | 90-95% recovery in closed-loop systems [91] | Energy-intensive distillation required | Reduces raw material consumption and energy use |
| Process Waste | Minimal; only exhausted raw material [20] | Contaminated solvents and adsorbents | Eliminates hazardous waste disposal challenges |
| Carbon Footprint | Lower due to solvent recycling | Higher from solvent production and disposal | Contributes to climate change mitigation |
| Energy Intensity | Moderate (compression energy) | High (distillation, solvent production) | Reduced overall energy consumption |
Beyond the direct advantages in solvent use, SC-COâ‚‚ systems demonstrate superior environmental performance through their operational characteristics. The technology enables higher selectivity and extraction efficiency, reducing the need for downstream purification steps that typically generate additional waste [20]. Furthermore, the adaptability of SC-COâ‚‚ to continuous processing modes enhances energy efficiency compared to batch-oriented traditional methods [90]. The carbon dioxide used in these processes is predominantly sourced from existing industrial byproducts, further improving the overall environmental lifecycle assessment [20].
Carbon Dioxide Lifecycle Analysis
The environmental assessment of SC-COâ‚‚ extraction must consider the complete lifecycle of the carbon dioxide solvent. While the direct environmental impact is minimal due to the non-toxic nature of COâ‚‚, the sourcing and energy requirements for compression contribute to the overall environmental footprint. Most commercial SC-COâ‚‚ systems utilize COâ‚‚ captured as a byproduct from other industrial processes such as fermentation, ammonia production, or fossil fuel combustion, thereby avoiding the dedicated production of extraction solvents [20].
The energy intensity of SC-COâ‚‚ extraction primarily stems from the compression of COâ‚‚ to supercritical conditions. Modern systems incorporate energy recovery mechanisms during depressurization stages, significantly improving overall efficiency [90]. Additionally, technological advancements in system design, including multi-stage separation and heat integration, have further reduced energy consumption per unit of extract produced [90]. When properly optimized, SC-COâ‚‚ systems demonstrate a favorable environmental profile across multiple impact categories, including global warming potential, ecotoxicity, and human health impacts, compared to solvent-intensive alternatives.
Regulatory Compliance Framework
International Regulatory Landscape
The regulatory environment increasingly favors SC-COâ‚‚ extraction technology due to its alignment with global initiatives promoting green chemistry and sustainable manufacturing. International regulatory bodies have established stringent limitations on residual solvents in pharmaceutical, food, and cosmetic products, creating a significant advantage for SC-COâ‚‚-based processes that eliminate this concern entirely [20] [90]. The Generally Recognized as Safe (GRAS) status of carbon dioxide by food safety authorities worldwide facilitates the adoption of SC-COâ‚‚ extraction for food and nutraceutical applications without complex regulatory approvals for solvent residues [20].
Table 2: Regulatory Status and Advantages of SC-COâ‚‚ Extraction Across Industries
| Industry | Regulatory Classification | Key Compliance Advantages |
|---|---|---|
| Pharmaceuticals | FDA acceptance of residual COâ‚‚ as harmless [20] | Eliminates ICH Q3C residual solvent testing; simplifies purification [92] |
| Food & Beverages | GRAS status worldwide [20] | "No-solvent" labeling claim; avoids solvent residue regulations |
| Cosmetics | Approved for "natural" and "organic" certifications [7] [20] | Complies with ISO 16128 natural origin standard; clean ingredient listing |
| Nutraceuticals | Meets FDA GMP for dietary supplements [20] | Eliminates solvent contamination concerns in complex regulatory landscape |
Region-specific regulatory trends further support SC-COâ‚‚ adoption. The European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) program imposes strict controls on volatile organic compound (VOC) emissions, directly favoring closed-loop SC-COâ‚‚ systems [90]. Similarly, the United States Environmental Protection Agency (EPA) has established increasingly stringent VOC emission standards that create compliance challenges for conventional solvent-based extraction facilities [7] [90]. These regulatory developments have prompted significant industry investment in SC-COâ‚‚ technology as a long-term compliance strategy.
Environmental Regulations and Compliance
SC-COâ‚‚ extraction technology provides inherent advantages in complying with environmental regulations governing industrial emissions and waste management. The process significantly reduces or eliminates reports under the EPA's Toxic Release Inventory (TRI) program that typically apply to facilities using substantial quantities of regulated solvents [90]. Furthermore, the technology minimizes liabilities associated with solvent storage, handling, and disposal regulations under the Resource Conservation and Recovery Act (RCRA) [90].
The closed-loop design of modern SC-COâ‚‚ systems prevents atmospheric emissions of VOCs, helping facilities comply with Clean Air Act requirements [90]. This is particularly valuable in non-attainment areas where VOC emissions are strictly limited. Additionally, the absence of contaminated wastewater streams from solvent recovery operations eliminates permitting requirements under the Clean Water Act and reduces compliance monitoring costs [20] [90]. These regulatory advantages translate to reduced compliance burden, lower operating costs, and diminished environmental liability for facilities implementing SC-COâ‚‚ technology.
Experimental Protocols for Environmental and Compliance Testing
Standardized Extraction Methodology
To ensure consistent environmental and regulatory performance, researchers should adhere to standardized experimental protocols for SC-COâ‚‚ extraction. The following methodology outlines a comprehensive approach for extraction process optimization and environmental impact assessment:
Raw Material Preparation: The biological material (e.g., plant matter) should be dried to optimal moisture content (typically 5-10%) and ground to consistent particle size (0.2-0.8 mm) to ensure efficient mass transfer while minimizing energy consumption [91] [16]. Excessive moisture reduces extraction efficiency and increases energy requirements for water removal [16].
System Preparation and Equilibration: The extraction vessel should be packed uniformly to avoid channeling, which reduces efficiency. The system is then pressurized and heated to the desired operating conditions. Typical analytical-scale operations use vessels of 50-500 mL capacity, while industrial systems may exceed 100 L [38] [91].
Extraction Process Optimization: Utilizing Design of Experiments (DoE) methodology, particularly Response Surface Methodology (RSM), systematically varies pressure (25-45 MPa), temperature (40-60°C), and extraction time (10-110 minutes) to determine optimal parameters for maximum yield with minimal energy input [91].
Separation and Collection: Implement fractional separation using multiple separators in series at progressively lower pressures to fractionate extracts without additional purification steps, reducing overall energy consumption [20].
Solvent Recycling: The COâ‚‚ is condensed and recycled back to the storage vessel for reuse, with recycling rates typically reaching 90-95% in optimized systems [91].
This protocol should be documented with comprehensive process parameters to support regulatory submissions and environmental impact assessments.
Analytical Methods for Compliance Verification
Robust analytical methods are essential for verifying regulatory compliance and environmental performance of SC-COâ‚‚ extraction processes:
Residual Solvent Analysis: Despite the absence of organic solvents, validated gas chromatography-mass spectrometry (GC-MS) methods should confirm the lack of solvent residues in final extracts, meeting pharmacopeial requirements [20].
Extract Purity and Composition: Employ ultra-high performance liquid chromatography (uHPLC) with diode array detection to quantify target compounds and potential co-extractives. For cannabinoid analysis, a validated method separating 11 cannabinoids in a single run has been demonstrated [93].
Environmental Emission Monitoring: Implement continuous monitoring of COâ‚‚ emissions and periodic verification of closed-system integrity to confirm minimal environmental release [90].
Energy Consumption Assessment: Standardized measurement of energy input per unit of extract produced (kWh/kg) provides data for comparative environmental assessments [90].
These analytical protocols generate the necessary data for regulatory submissions and environmental impact reporting.
Technological Implementation and Optimization
Advanced Process Optimization Techniques
Modern SC-CO₂ extraction benefits from advanced optimization approaches that enhance both environmental performance and regulatory compliance. Response Surface Methodology (RSM) with Box-Behnken or Central Composite Designs enables efficient parameter optimization with minimal experimental runs, reducing resource consumption during process development [91]. For instance, paprika extraction optimization demonstrated maximum yield (10.05%) and carotenoid content (4.21%) at 45 MPa, 50°C, and 74 minutes through RSM [91].
Machine learning algorithms now offer powerful tools for predictive optimization without extensive experimental trials. Recent research demonstrates that convolutional neural networks (CNN) can predict drug solubility in SC-CO₂ with high accuracy (R² = 0.9839), using molecular weight, melting point, pressure, and temperature as inputs [76]. These computational approaches significantly reduce the environmental footprint of process development while ensuring optimal parameter selection for regulatory compliance.
System Design for Enhanced Sustainability
Innovations in SC-COâ‚‚ system design further improve environmental performance and regulatory alignment. Modern implementations incorporate:
- Energy Recovery Systems: Technologies that capture energy during depressurization stages significantly reduce net energy consumption [90].
- Advanced Separator Design: Specially designed separating chambers immediately following the extraction vessel reduce throttling effects and dry ice formation during depressurization, improving efficiency and yield [93].
- Continuous Processing: Moving from batch to continuous extraction modes enhances throughput and reduces energy consumption per unit product [90].
- Modular System Architecture: Enables scalability from laboratory to industrial scale with consistent environmental and regulatory performance [90].
These design innovations support the economic viability and sustainability of SC-COâ‚‚ technology across diverse applications.
Visualization of SC-COâ‚‚ Extraction Workflow
The following diagram illustrates the complete SC-COâ‚‚ extraction process, highlighting environmental and regulatory advantages at each stage:
SC-COâ‚‚ Extraction Environmental & Regulatory Advantages
This workflow visualization highlights how the intrinsic design of SC-COâ‚‚ extraction systems incorporates environmental and regulatory advantages at each process stage, from the use of GRAS (Generally Recognized as Safe) solvents to closed-loop recycling that minimizes emissions.
Essential Research Reagent Solutions
Implementation of SC-COâ‚‚ extraction requires specific materials and reagents optimized for supercritical operations. The following table details essential research reagent solutions for establishing compliant SC-COâ‚‚ extraction protocols:
Table 3: Essential Research Reagents for SC-COâ‚‚ Extraction
| Reagent/Material | Technical Specification | Function in SC-COâ‚‚ Extraction |
|---|---|---|
| Carbon Dioxide | 99.9% purity, food/pharma grade [91] | Primary supercritical solvent; high purity ensures extract quality and regulatory compliance |
| Co-solvents | Ethanol (95-99%), methanol, water (HPLC grade) [20] | Polarity modifiers for enhanced extraction of polar compounds; ethanol preferred for regulatory acceptance |
| Reference Standards | Certified reference materials for target compounds [93] | Quantification and method validation; essential for regulatory submissions |
| Biomass Preparation | Diatomaceous earth, glass beads (3mm) [38] | Matrix modifiers that prevent caking and improve COâ‚‚ contact with biomass |
| Filter Materials | Metal frit filters (5μm pore size) [38] | Prevent biomass transfer to extraction lines while maintaining flow |
| Calibration Mixtures | 37 FAME mix, cannabinoid standards [38] [93] | System calibration and quantitative analysis |
| Extraction Vessels | 50mL-100L capacity, 250-680 bar rating [38] [91] | Contain biomass during extraction; pressure rating determines operational range |
These research reagents form the foundation for establishing robust, reproducible, and compliant SC-COâ‚‚ extraction methods across various applications.
Supercritical COâ‚‚ extraction technology represents a convergence of environmental sustainability and regulatory compliance in industrial separation processes. The method's elimination of toxic solvents, minimal waste generation, and reduced energy consumption position it as a cornerstone technology for green chemistry initiatives across pharmaceutical, food, and cosmetic industries [20] [90]. Simultaneously, the regulatory advantages stemming from the GRAS status of COâ‚‚ and elimination of solvent residue concerns provide significant compliance benefits in increasingly stringent regulatory environments [7] [20] [90].
The ongoing technological advancements in SC-COâ‚‚ system design, process optimization, and analytical verification continue to enhance both environmental performance and regulatory alignment. The integration of machine learning for solubility prediction [76], advanced process control for energy optimization [90], and comprehensive analytical methods for quality verification [93] collectively support the implementation of SC-COâ‚‚ technology as a sustainable and compliant extraction solution. For researchers and drug development professionals, mastery of SC-COâ‚‚ extraction principles, environmental implications, and regulatory considerations provides a significant strategic advantage in developing next-generation products aligned with global sustainability and quality standards.
Analysis of Final Product Purity, Solvent Residues, and Thermolabile Compound Preservation
Supercritical carbon dioxide (SC-CO2) extraction has emerged as a pivotal green technology within pharmaceutical and nutraceutical research, addressing critical challenges in final product purity, solvent residue elimination, and thermolabile compound preservation. This technique utilizes carbon dioxide above its critical temperature (31.1°C) and pressure (73.8 bar), creating a solvent with unique properties that are tunable for specific extraction needs [10] [20]. The pharmaceutical industry faces significant hurdles in eliminating organic solvent residues from active pharmaceutical ingredients (APIs), as these solvents are classified by toxicity into three categories, with Class 1 being the most toxic and Class 3 the lowest risk [94]. Furthermore, conventional extraction methods often degrade thermolabile bioactive compounds through excessive heat exposure or leave behind toxic solvent residues that compromise product safety and efficacy [10] [20]. SC-CO2 extraction provides a robust solution to these challenges, offering researchers a methodology that aligns with green chemistry principles while maintaining stringent purity standards required for pharmaceutical applications [10] [95]. This technical guide examines the fundamental mechanisms, experimental parameters, and methodological protocols that underpin the superior performance of SC-CO2 extraction in these three critical areas, providing researchers with a comprehensive framework for implementation within their investigative workflows.
Fundamental Principles of Supercritical CO2 Extraction
Unique Physicochemical Properties
Supercritical CO2 exhibits hybrid physicochemical properties that make it particularly suitable for pharmaceutical extraction applications. In its supercritical state, CO2 possesses liquid-like densities (200-900 kg/m³) enabling substantial solvating power, while maintaining gas-like low viscosities (0.01-0.09 cP) and high diffusivities (0.01-0.001 cm²/s) that facilitate rapid mass transfer and penetration into complex matrices [10]. This combination allows for efficient extraction while operating at moderate temperatures that preserve thermolabile compounds. The tunable solvent power of SC-CO2 represents its most technologically significant attribute, as solvent density can be precisely manipulated through controlled adjustments of pressure and temperature parameters [10] [20]. For instance, density increases with pressure at constant temperature, enhancing solvating power for larger molecules, while density decreases with temperature at constant pressure, providing a mechanism for selective fractionation [10]. This tunability enables researchers to selectively target specific compound classes through systematic parameter optimization, making SC-CO2 an exceptionally versatile extraction platform for complex natural product matrices.
Mechanism of Thermolabile Compound Preservation
The preservation of thermolabile compounds in SC-CO2 extraction operates through two primary mechanisms: low operational temperatures and the absence of oxidative degradation. Unlike conventional extraction methods that often employ temperatures exceeding 80-100°C, SC-CO2 extractions typically occur between 31-60°C, well below the degradation threshold for most heat-sensitive compounds like flavonoids, essential oils, and unsaturated fatty acids [20] [9]. Furthermore, CO2 provides an inert environment that prevents oxidative degradation during the extraction process, particularly crucial for compounds prone to oxidation such as carotenoids and polyunsaturated lipids [10]. The supercritical state facilitates rapid diffusion and extraction kinetics, significantly reducing processing time and further minimizing potential thermal exposure [20]. Research demonstrates that SC-CO2 extracted essential oils maintain higher concentrations of volatile aromatic compounds and exhibit superior biological activity compared to those obtained through steam distillation or solvent extraction [28] [55]. This preservation efficacy extends to various thermolabile pharmaceutical compounds, including antibiotics, vitamins, and phytocannabinoids, making SC-CO2 particularly valuable for extracting bioactive molecules with defined therapeutic applications.
Quantitative Analysis of Final Product Purity and Solvent Residues
Comparative Solvent Residue Profiles
The capacity of SC-CO2 extraction to eliminate organic solvent residues from final products represents one of its most significant advantages for pharmaceutical applications. Conventional extraction methodologies frequently employ Class 2 and Class 3 solvents which can persist in APIs despite rigorous purification protocols. Research demonstrates that SC-CO2 extraction achieves remarkable efficiency in reducing these residual solvents to levels substantially below regulatory thresholds [94].
Table 1: Solvent Residue Reduction in APIs via Supercritical CO2 Extraction
| Active Pharmaceutical Ingredient | Solvent | Initial Concentration (ppm) | Post-SFE Concentration (ppm) | Reduction Efficiency (%) |
|---|---|---|---|---|
| Beclometasone dipropionate | Ethanol | 4200 | 0 | 100 |
| Beclometasone dipropionate | n-Heptane | 310 | 38 | 87.7 |
| Beclometasone dipipropionate | Ethyl acetate | 890 | 52 | 94.2 |
| Budesonide | Ethanol | 3200 | 0 | 100 |
| Budesonide | n-Heptane | 270 | 35 | 87.0 |
| Budesonide | Toluene | 70 | 19 | 72.9 |
Data adapted from Baldino et al. [94]
As evidenced in Table 1, SC-CO2 extraction completely eliminated ethanol residues from both corticosteroid APIs and substantially reduced other Class 2 and 3 solvents. This elimination capability stems from the complete miscibility of many organic solvents with SC-CO2 at moderate pressures (90-100 bar) and temperatures (40°C) [94]. The miscibility creates a homogeneous phase where solvent molecules partition preferentially into the SC-CO2 stream, facilitating their efficient removal from the solid API matrix. This process occurs without exposing the API to high temperatures that might cause decomposition or denaturation, addressing a significant limitation of conventional drying techniques [94].
Purity Enhancement Through Selective Extraction
The tunable solvent power of SC-CO2 enables unprecedented selectivity in extraction processes, directly contributing to enhanced final product purity. By strategically manipulating pressure and temperature parameters, researchers can target specific compound classes while excluding undesirable constituents that would co-extract using conventional solvents.
Table 2: Purity Optimization Through Parameter Modulation in SFE
| Target Compound | Plant Source | Optimal Pressure (bar) | Optimal Temperature (°C) | Purity Achieved (%) | Comparison with Conventional Methods (%) |
|---|---|---|---|---|---|
| Artemisinin | Artemisia annua | 200-300 | 40-50 | 90-92 | 70-75 (Ethanol extraction) |
| Carnosic acid | Rosemary leaves | 250-300 | 50-60 | 95 | 72 (Ethanol extraction) |
| Essential oils | Hetian rose | 350 | 40 | 98.5 | 85-90 (Steam distillation) |
| Curcumin | Turmeric | 250-300 | 50-60 | 95 | 80 (Acetone extraction) |
| Lycopene | Tomato | 300-400 | 60-70 | 90 | 70 (Hexane extraction) |
Data compiled from multiple sources [41] [9] [55]
The precision extraction demonstrated in Table 2 highlights how SC-CO2 parameters can be optimized to isolate target compounds with minimal co-extraction of impurities. This selectivity reduces downstream purification requirements and eliminates the need for additional processing steps that might compromise product yield or compound integrity. The exceptional purity of SC-CO2 extracts directly translates to enhanced biological activity and therapeutic efficacy, as evidenced by higher antioxidant and antimicrobial activities compared to conventionally extracted materials [9] [55].
Experimental Protocols for Optimal Extraction
Standardized SFE Protocol for Thermolabile Compounds
The following protocol provides a methodological framework for extracting thermolabile compounds while preserving their structural integrity and bioactivity:
Sample Preparation: Reduce plant material to particle size of 0.3-0.8 mm to enhance mass transfer while preventing channeling. Maintain moisture content below 10% to prevent ice formation and extraction interference [16] [55]. For rose essential oil extraction, optimal particle size was determined to be 0.8 mm [55].
Extraction Vessel Loading: Pack extraction vessel evenly to ensure homogeneous flow distribution and prevent channeling. Incorporate inert packing materials like glass beads at both ends of the vessel to promote uniform fluid distribution.
System Pressurization and Heating: Gradually increase pressure to desired setpoint (typically 100-350 bar) followed by temperature adjustment to target value (35-70°C). Maintain temperature stability within ±1°C and pressure within ±2 bar throughout extraction [20].
Dynamic Extraction: Initiate CO2 flow at predetermined rate (1-10 kg/h depending on scale). For rose essential oil, optimal flow rate was established at 10 L/h [55]. Maintain extraction parameters for predetermined duration based on breakthrough curves.
Separation and Collection: Depressurize CO2-extract mixture through back-pressure regulator into separation vessel maintained at lower pressure (50-80 bar) and temperature (15-25°C) to precipitate extracts. For fractionated collection, employ multiple separators in series with descending pressure thresholds [20].
CO2 Recycling: Condense and recycle CO2 in closed-loop systems to reduce operational costs and environmental impact, achieving up to 95% recycling efficiency [41].
Solvent Residue Elimination Protocol
This specialized protocol targets the removal of organic solvent residues from pre-processed APIs:
API Characterization: Determine initial solvent residue profile via GC-FID analysis. Identify all Class 1, 2, and 3 solvents present and their respective concentrations [94].
Extraction Vessel Preparation: Load API into extraction vessel, ensuring uniform packing density. For laboratory-scale systems, typical loadings range from 5-50 g.
Optimized Parameter Selection: Set pressure to 100-200 bar and temperature to 40°C, conditions sufficient to reach mixture critical points with most organic solvents [94].
Extended Static Extraction: Implement static extraction mode for 30-60 minutes to allow complete interaction between SC-CO2 and solvent residues within the API matrix.
Dynamic Purge Phase: Initiate CO2 flow at 1-2 kg/h for 2-4 hours to remove solubilized solvent residues from the system.
Post-Processing Analysis: Conduct GC-FID analysis to verify residue reduction. For stubborn solvents like toluene, consider a second extraction cycle at slightly elevated pressure [94].
Enhanced Protocols with Co-Solvents
For polar compounds requiring solubility enhancement, modified protocols with co-solvents are employed:
Co-solvent Selection: Choose appropriate co-solvent based on target compound polarity. Food-grade ethanol is preferred for pharmaceutical applications at concentrations of 1-15% (v/v) [20] [9].
Co-solvent Introduction: Introduce co-solvent via separate pump system to ensure precise concentration control. Premixing with matrix is an alternative for batch processes.
Parameter Adjustment: Adjust pressure and temperature to accommodate modified solvent system. Typically, lower pressures are required when using co-solvents compared to pure SC-CO2.
Co-solvent Removal: Implement stepwise pressure reduction in separation vessels to recover co-solvent separately from target compounds when necessary.
Visualization of SFE Process and Mechanisms
SFE System Workflow
SFE System Schematic - This diagram illustrates the closed-loop workflow of supercritical COâ‚‚ extraction systems, highlighting the transformation of COâ‚‚ and its interaction with plant material or APIs throughout the process.
Purity Preservation Mechanism
Purity Preservation Pathways - This diagram outlines the fundamental mechanisms through which supercritical COâ‚‚ extraction preserves compound integrity and ensures final product purity.
The Researcher's Toolkit: Essential Materials and Reagents
Table 3: Essential Research Equipment and Reagents for SFE
| Item | Specification | Function | Application Notes |
|---|---|---|---|
| Supercritical Fluid Extractor | Laboratory-scale system (1-5L), pressure rating 400-500 bar, temperature range 20-100°C | Primary extraction apparatus | Ensure safety certifications; prefer modular systems for flexibility [20] |
| Food-Grade COâ‚‚ | 99.9% purity, with dip tube for liquid withdrawal | Primary extraction solvent | Higher purity reduces system contamination and analytical interference [55] |
| Co-solvents | HPLC-grade ethanol, methanol (≥99.9%) | Polarity modification for enhanced extraction of polar compounds | Ethanol preferred for pharmaceutical applications due to GRAS status [9] |
| Analytical GC-FID | Flame ionization detector, capillary columns | Solvent residue analysis in APIs | Regular calibration with certified standards essential [94] |
| Cellulase/Pectinase Enzymes | Food-grade, activity ≥99% | Sample pretreatment for cell wall disruption | Optimize concentration (2-10%) and incubation time for specific matrices [55] |
| Sample Preparation Equipment | Mechanical mill (particle size 0.3-1.2 mm), moisture analyzer | Matrix preparation for optimal extraction | Controlled particle size critical for reproducible mass transfer rates [55] |
Supercritical CO2 extraction represents a technologically advanced platform that directly addresses the pharmaceutical industry's critical requirements for enhanced final product purity, elimination of solvent residues, and preservation of thermolabile compounds. Through its tunable physicochemical properties and operational flexibility, SC-CO2 enables researchers to achieve extraction efficiencies and product qualities unattainable through conventional methodologies. The quantitative data presented in this analysis demonstrates the capacity of SC-CO2 to completely eliminate certain solvent residues while substantially reducing others to levels well below regulatory thresholds. Furthermore, the temperature-sensitive nature of many bioactive compounds necessitates the mild operational conditions afforded by SC-CO2, which preserves molecular integrity and biological activity. As pharmaceutical research continues to emphasize green chemistry principles and sustainable manufacturing practices, SC-CO2 extraction stands as a cornerstone technology that aligns with these evolving priorities while delivering superior analytical and therapeutic outcomes. The experimental frameworks and technical parameters outlined in this guide provide researchers with a comprehensive foundation for implementing SC-CO2 methodologies within their investigative workflows, potentially accelerating the development of purer, safer, and more efficacious pharmaceutical products.
Market Growth and Future Trends in Pharmaceutical SFE-CO2 Adoption
Supercritical Fluid Extraction using carbon dioxide (SFE-CO₂) represents a transformative technology in pharmaceutical manufacturing, aligning with the global industry's shift toward greener and more efficient processes. This technique utilizes carbon dioxide above its critical temperature (31.1 °C) and pressure (7.38 MPa), where it exhibits unique properties ideal for extracting delicate bioactive compounds: gas-like diffusivity and viscosity coupled with liquid-like density [96] [20]. The pharmaceutical industry is increasingly adopting SFE-CO₂ to obtain high-purity active pharmaceutical ingredients (APIs) and natural bioactive compounds from plant materials, driven by its solvent-free nature, selectivity, and compliance with stringent regulatory standards [70] [20]. Within the context of foundational SFE-CO₂ research, this whitepaper examines the compelling growth dynamics, presents scalable experimental protocols, and projects the evolving role of this technology in developing next-generation pharmaceuticals, providing researchers and drug development professionals with a comprehensive technical guide.
Market Landscape and Growth Drivers
Quantitative Market Outlook
The global market for supercritical COâ‚‚ extraction equipment is experiencing robust growth, underpinned by rising demand across the pharmaceutical, nutraceutical, and cannabis-derived medicine sectors. The table below summarizes key market projections and regional dynamics based on recent analyses.
Table 1: Supercritical COâ‚‚ Extraction Equipment Market Projections
| Region | Market Size (2023/2024) | Projected Market Size (2032/2033) | CAGR | Primary Growth Drivers |
|---|---|---|---|---|
| Global | USD 1.2 billion [97] | USD 2.5 billion by 2032 [97] | 7.8% [97] | Demand for natural/organic products, stringent regulations, environmental concerns [97] |
| Global (Machines) | USD 0.072 Billion (2024) [98] | USD 0.105 Billion by 2033 [98] | 7.7% [98] | Not specified |
| United States | USD 7.49 billion (2025) [99] | USD 17.43 billion by 2033 [99] | 15.12% [99] | Technological innovation, expanding legal cannabis industry, consumer demand for natural products [99] |
Table 2: Regional Market Dynamics and Application Segmentation
| Region | Market Share & Growth | Key Industries Driving Demand |
|---|---|---|
| North America | Significant share and early adoption [70] [97] | Advanced pharmaceuticals, legal cannabis, nutraceuticals [70] [72] |
| Europe | Substantial market, stringent regulations promote adoption [70] [97] | Pharmaceutical research, green chemistry initiatives, natural cosmetics [70] |
| Asia-Pacific | Fastest-growing region [70] [97] | Expanding pharmaceutical and nutraceutical sectors, government innovation grants [70] [72] |
| Application | Market Leadership & Potential | Key Extracted Components |
| Pharmaceuticals | Leading application segment [70] [72] | High-purity APIs, medicinal plant bioactives [70] [96] |
| Nutraceuticals | Projected high growth segment [72] | Herbal extracts, health supplements, potent bioactive compounds [70] [72] |
Key Growth Drivers in Pharmaceutical Adoption
Several interconnected factors are accelerating the adoption of SFE-COâ‚‚ within pharmaceutical manufacturing:
Regulatory Pressure and Green Chemistry Principles: Strict international standards, such as Good Manufacturing Practices (GMP), favor solvent-free methods for producing high-purity compounds [70]. The design of "greener APIs" is increasingly feasible by integrating environmental parameters into drug R&D, a paradigm supported by pharmaceutical industry experts [100]. SFE-COâ‚‚ aligns perfectly with this approach, using a generally recognized as safe (GRAS) solvent and eliminating concerns about toxic solvent residues in final drug products [20] [50].
Consumer and Market Shift Toward Natural Products: Growing consumer preference for clean-label, natural, and organic products pushes industries to adopt cleaner extraction methods [70] [72]. SFE-COâ‚‚ delivers residue-free extracts ideal for pharmaceuticals, nutraceuticals, and cosmeceuticals, meeting the demand for both purity and "natural" provenance [70].
Technological and Economic Advancements: While initial capital investment is high, large-scale SFE-COâ‚‚ systems lower per-unit processing costs, offering attractive long-term returns [70]. Innovations in automation, AI-driven process optimization, and modular system design are improving yields, reducing operational costs, and making the technology more accessible [70] [97].
Technical Protocols and Workflow Optimization
Core SFE-COâ‚‚ System Operation
A standard SFE-COâ‚‚ system for pharmaceutical extraction consists of several key components that create a continuous workflow. The process begins with cooling and pressurization of COâ‚‚, followed by extraction and final separation of the target compounds.
Optimized Protocol for Cannabinoid Extraction
The following protocol, optimized using Design of Experiments (DOE) methodology, demonstrates the scalable extraction of pharmaceutical-grade cannabinoids from 1 kg of cured cannabis plant material [50]. This approach systematically evaluates critical parameters to maximize yield and purity.
Table 3: Research Reagent Solutions and Essential Materials
| Item | Specification/Function |
|---|---|
| Raw Material | 1 kg cured, milled cannabis biomass (e.g., THC:CBD ≈ 1:1.5) [50] |
| Extraction Solvent | Food-grade or higher purity COâ‚‚ (serves as supercritical solvent) [50] |
| Co-solvent (Optional) | Anhydrous ethanol or methanol (enhances polar compound solubility) [20] |
| Reference Standards | Certified THC, CBD, CBDA, THCA standards for HPLC calibration and quantification [50] |
Pre-Extraction Sample Preparation:
- Milling: Reduce dried cannabis bud material to a consistent particle size (e.g., 0.5-1 mm) to increase surface area and minimize mass transfer resistance [50].
- Curing/Decarboxylation: Heat the biomass (e.g., 105-120 °C for 1-2 hours) to convert acid cannabinoid precursors (THCA, CBDA) to their active neutral forms (THC, CBD) [50].
- Vessel Packing: Uniformly pack the biomass into the extraction vessel, avoiding channeling that can reduce extraction efficiency [50].
DOE-Optimized Extraction Procedure:
- System Initialization: Set the extraction vessel temperature to 60 °C. Pressurize the system to the target pressure (e.g., 320 bar) using the high-pressure pump [50].
- Dynamic Extraction: Initiate COâ‚‚ flow at the optimized rate of 150 g/min. Maintain a constant extraction time of 600 minutes [50].
- Separation and Collection: In the separator vessel, reduce pressure (typically to 50-60 bar) to decrease COâ‚‚ solvent power and precipitate the extract. Collect the crude extract gravimetrically [50].
- Solvent Recycling: Re-liquefy the COâ‚‚ from the separator using a chiller and return it to the COâ‚‚ reservoir for reuse, creating a closed-loop system [50].
Analytical Quantification:
- HPLC Analysis: Use High-Performance Liquid Chromatography (HPLC) with UV detection to quantify cannabinoid content (THC, CBD) in the extract. Compare against certified reference standards to determine recovery efficiency [50].
- Yield Calculation: Calculate extraction yield as (mass of crude extract / mass of initial biomass) × 100. Calculate cannabinoid recovery as (mass of cannabinoid in extract / theoretical mass in biomass) × 100 [50].
Parameter Optimization Strategy
The DOE approach revealed that COâ‚‚ flow rate exerts the most significant influence on CBD recovery, followed by extraction time, while pressure had the least individual impact [50]. The optimized parameters for maximum yield (7.1%) and cannabinoid recovery were: high flow rate (150 g/min), long extraction time (600 min), and high pressure (320 bar) [50]. The following diagram illustrates the experimental optimization workflow and the relative impact of each parameter based on the DOE findings.
Future Trends and Research Directions
The future of SFE-COâ‚‚ in pharmaceuticals is being shaped by several convergent technological and strategic trends:
Process Intensification and Advanced Controls: Integration of Artificial Intelligence (AI) and Machine Learning (ML) for real-time process control is emerging as a key innovation. AI-driven systems can dynamically optimize extraction parameters (pressure, temperature, flow rate, modifier addition) to maximize yield and selectivity for target compounds, moving beyond static protocols [70]. Furthermore, hybrid systems that combine SFE with other techniques like ultrasound or microwave assistance are under development to enhance extraction efficiency and reduce energy consumption [70] [20].
Expansion into New Pharmaceutical Domains: Beyond botanical extraction, SFE-COâ‚‚ is finding novel applications in drug formulation. The technology is pivotal in particle engineering techniques, such as the Rapid Expansion of Supercritical Solutions (RESS), to produce micro- and nano-particles of APIs with controlled particle size and crystallinity, improving drug solubility and bioavailability [84]. This aligns with the industry's need for consistent production of solid drug materials under cGMP conditions [84].
Sustainability and Green Pharmacy Integration: The role of SFE-CO₂ is expanding within the "benign by design" API framework, which aims to minimize the environmental impact of pharmaceuticals throughout their lifecycle [100]. The technology's inherent green attributes—using non-toxic, recyclable CO₂ and generating no hazardous solvent waste—make it a cornerstone for sustainable drug development. Future research will focus on integrating environmental impact assessments early in the R&D process, leveraging SFE-CO₂ to achieve both efficacy and environmental sustainability [100].
SFE-COâ‚‚ technology has matured into a cornerstone of modern, sustainable pharmaceutical extraction. Driven by compelling market growth, rigorous technical optimization via methodologies like DOE, and alignment with global green chemistry principles, its adoption is set to accelerate. For researchers and drug development professionals, mastering this technology is no longer optional but essential. The future lies in leveraging its versatility not just for isolation, but for creating novel drug formulations and fully embracing the principles of green pharmacy, ultimately leading to cleaner, safer, and more efficient pharmaceutical production.
Conclusion
Supercritical CO2 extraction presents a powerful, sustainable alternative to traditional solvent-based methods, offering unparalleled selectivity, enhanced purity, and superior preservation of heat-sensitive bioactive compounds crucial for pharmaceutical development. The successful implementation of SFE-CO2 hinges on a deep understanding of its foundational principles and the strategic optimization of key parameters. As machine learning models advance in predicting drug solubility and process efficiency, SFE-CO2 is poised to revolutionize pharmaceutical manufacturing. Its role is expected to expand significantly in green drug processing, advanced particle engineering for drug delivery systems, and the purification of complex active pharmaceutical ingredients (APIs), solidifying its position as a cornerstone technology for the future of sustainable and precise pharmaceutical research and production.
References
- 1. Supercritical carbon dioxide - Wikipedia [https://en.wikipedia.org/wiki/Supercritical_carbon_dioxide]
- 2. Critical point of carbon dioxide: [https://pages.mtu.edu/~rluck/courses/spring2004/spring2004CH1110/lecture/Criticalco2.htm]
- 3. Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4206850/]
- 4. Why is carbon dioxide used most often in SFE? [https://www.supercriticalfluids.com/why-is-carbon-dioxide-used-most-often-in-sfe/]
- 5. High Fidelity Analysis of Advanced Turbines for Zero ... [https://hammer.purdue.edu/articles/thesis/High_Fidelity_Analysis_of_Advanced_Turbines_for_Zero_Emission_Supercritical_CO2_Cycles/27214467]
- 6. Critical State of Carbon Dioxide — Collection of Experiments [https://physicsexperiments.eu/1771/critical-state-of-carbon-dioxide]
- 7. Supercritical Carbon Dioxide (CO2) Extraction [https://www.archivemarketresearch.com/reports/supercritical-carbon-dioxide-co2-extraction-495272]
- 8. Thermodynamic properties of supercritical carbon dioxide ... [https://www.sciencedirect.com/science/article/pii/S2949821X25000274]
- 9. Supercritical Extraction Techniques for Obtaining ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11171758/]
- 10. Solvent Supercritical Fluid Technologies to Extract ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6152233/]
- 11. All the Advantages of Supercritical CO2 Extraction [https://separeco.com/articles/all-the-advantages-of-supercritical-co2-extraction-the-new-method-that-combines-quality-and-safety/]
- 12. The Advantages of Supercritical CO2 [https://www.azom.com/article.aspx?ArticleID=20458]
- 13. Supercritical CO2 Extraction [https://www.superex.com.tr/en/supercritical-extraction/introduction/]
- 14. Supercritical CO2 Extraction [https://www.phasex4scf.com/supercritical-co2-extraction]
- 15. Supercritical Extraction and Fractional Separation - [https://www.equilibar.com/application/supercritical-fractionation-pressure-control/]
- 16. Carbon Dioxide Supercritical Extraction - an overview [https://www.sciencedirect.com/topics/engineering/carbon-dioxide-supercritical-extraction]
- 17. Supercritical CO2 extraction of artemisinin from Artemisia ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12159492/]
- 18. Supercritical fluid extraction [https://en.wikipedia.org/wiki/Supercritical_fluid_extraction]
- 19. Steps Involved in a Supercritical CO2 Extraction Process [https://www.buffaloextracts.com/knowledge/what-are-the-steps-involved-in-a-supercritical-co2-extraction-process-here-is-your-guide/]
- 20. Recent Advances in Supercritical Fluid Extraction of Natural ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7504334/]
- 21. Process optimization for the supercritical carbondioxide ... [https://www.nature.com/articles/s41598-021-89772-6]
- 22. Modeling and optimization of supercritical fluid extraction of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4152520/]
- 23. The 2025 International Conference on Supercritical Carbon ... [https://icspc.allconfs.com/index_en.asp?id=A4458B4C3D25D397897E178E7C1D11A7]
- 24. Supercritical-CO2 extraction, identification and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8018978/]
- 25. SFE Process - Supercritical CO2 equipment solutions [https://sfe-process.com/]
- 26. Fundamentals of Supercritical Fluid Extraction: Using ... [https://www.cannabissciencetech.com/view/fundamentals-of-supercritical-fluid-extraction-using-carbon-dioxide-as-an-environmentally-friendly-solvent-for-extraction-of-cannabis]
- 27. Types of Supercritical CO2 Extraction Processes [https://www.buffaloextracts.com/knowledge/supercritical-co2-extraction-processes/]
- 28. A Bibliometric Analysis of the Supercritical CO2 Extraction ... [https://www.mdpi.com/2311-7524/10/11/1185]
- 29. Supercritical carbon dioxide extraction of astaxanthin from ... [https://bioresourcesbioprocessing.springeropen.com/articles/10.1186/s40643-025-00882-9]
- 30. Effect of Supercritical Carbon Dioxide Extraction ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5484124/]
- 31. Supercritical CO2 extraction of grape seed oil [https://www.sciencedirect.com/science/article/pii/S089684461400432X]
- 32. Predicting drug solubility in supercritical carbon dioxide ... [https://www.nature.com/articles/s41598-025-24006-7]
- 33. Identification of a supercritical fluid extraction process for ... [https://www.sciencedirect.com/science/article/pii/S0360544222009367]
- 34. Supercritical CO2 extraction, chemical composition, and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9360582/]
- 35. Extraction, Isolation and Characterization of Bioactive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3218439/]
- 36. Conventional and modern techniques for bioactive ... [https://www.sciencedirect.com/science/article/pii/S2468227624004514]
- 37. Supercritical CO2 Extraction of Terpenoids from ... [https://www.mdpi.com/2304-8158/13/11/1719]
- 38. 3.4. CO2 Supercritical Extraction Experiments [https://bio-protocol.org/exchange/minidetail?id=5666375&type=30]
- 39. Extraction, Purification and Application of Bioactive ... [https://www.mdpi.com/journal/separations/special_issues/T30QSZ9EC6]
- 40. Supercritical Fluids: An Innovative Strategy for Drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11351119/]
- 41. Supercritical CO2 Extraction in Pharmaceuticals [https://www.buffaloextracts.com/knowledge/supercritical-co2-extraction-in-pharmaceuticals-driving-innovation-and-purity-standards/]
- 42. Particle synthesis by rapid expansion of supercritical ... [https://www.sciencedirect.com/science/article/abs/pii/S0021850221006741]
- 43. Supercritical fluid technology for solubilization of poorly ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8915588/]
- 44. Supercritical Anti Solvent Micronization (SAS) [https://separeco.com/co2-atomization-process/supercritical-antisolvent-micronization/]
- 45. Particles from Gas Saturated Solutions [https://hightechextracts.com/particles-from-gas-saturated-solutions/]
- 46. Computational analysis on the influence of pressure and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12623419/]
- 47. High-Fidelity Prediction of Drug Solubility in Supercritical ... [https://www.sciencedirect.com/science/article/pii/S0928098725003197]
- 48. Optimizing ethanol-modified supercritical COâ‚‚ extraction ... [https://www.nature.com/articles/s41598-025-91441-x]
- 49. Optimizing ethanol-modified supercritical COâ‚‚ extraction ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11904181/]
- 50. Utilisation of Design of Experiments Approach to Optimise ... [https://www.nature.com/articles/s41598-020-66119-1]
- 51. Development of a Validated LC-MS Method for the ... [https://www.mdpi.com/2076-3921/14/7/777]
- 52. Optimization of Supercritical Carbon Dioxide Fluid ... [https://www.mdpi.com/2227-9717/11/7/1953]
- 53. Biological activities in supercritical COâ‚‚-extracted cannabis [https://www.sciencedirect.com/science/article/abs/pii/S0896844625000932]
- 54. Ultrasonic-ethanol pretreatment assisted aqueous enzymatic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10818077/]
- 55. Research on the Supercritical CO2 Extraction Process of ... [https://www.mdpi.com/2227-9717/12/7/1396]
- 56. Factors Affecting CO2 Supercritical Fluid Extraction [https://www.toptiontech.com/info/factors-affecting-co2-supercritical-fluid-extr-103062716.html]
- 57. Optimizing CO2 Extraction Parameters [https://nisargabiotech.com/optimizing-co2-extraction-parameters/]
- 58. Effect of CO2 Flow Rate on the Extraction of Astaxanthin ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7766558/]
- 59. COâ‚‚ Extraction Efficiency: Pump Flowrate & Flow Control [https://www.buffaloextracts.com/knowledge/how-does-pump-flowrate-and-consistent-flow-control-affect-extraction-efficiency-in-co2-extraction-machines/]
- 60. Optimization of Supercritical Extraction Process, ... [https://pubmed.ncbi.nlm.nih.gov/40059563/]
- 61. Green Extraction of Plant Materials Using Supercritical CO2 [https://www.mdpi.com/2223-7747/13/16/2295]
- 62. Influence of particle size in supercritical carbon dioxide ... [https://www.nature.com/articles/s41598-023-32181-8]
- 63. Mathematical Modeling, Economic Evaluation and Scale-Up [https://www.mdpi.com/1420-3049/25/1/199]
- 64. Effect of supercritical carbon dioxide on pore structure and ... [https://www.sciencedirect.com/science/article/abs/pii/S0896844624001785]
- 65. Effect of Moisture and Oil Content in the Supercritical CO2 ... [https://www.mdpi.com/2304-8158/12/3/490]
- 66. Green Extraction of Plant Materials Using Supercritical CO2 [https://pmc.ncbi.nlm.nih.gov/articles/PMC11359946/]
- 67. Supercritical extraction with carbon dioxide and co-solvent ... [https://www.sciencedirect.com/science/article/abs/pii/S2214786118305710]
- 68. Effects of Ethanol on the Supercritical Carbon Dioxide ... [https://www.mdpi.com/2297-8739/8/9/154]
- 69. Effect of supercritical CO 2 modified with ethanol on the ... [https://www.sciencedirect.com/science/article/pii/S002364382401168X]
- 70. Global Market Growth for Supercritical Fluid Extraction [https://www.buffaloextracts.com/knowledge/global-demand-for-supercritical-fluid-extraction-equipment-market-growth-and-opportunities/]
- 71. Supercritical Carbon Dioxide + Ethanol Extraction to ... [https://www.mdpi.com/2227-9717/9/3/489]
- 72. Supercritical CO2 Extractor Market Driver [https://www.fortunebusinessinsights.com/supercritical-co2-extractor-market-112950]
- 73. Predicting drug solubility in supercritical carbon dioxide green ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12624000/]
- 74. Optimizing CO2 Extraction Parameters for Maximum Yield [https://nisargabiotech.com/optimizing-co2-extraction-parameters-for-maximum-yield/]
- 75. Machine learning estimation and optimization for ... [https://www.nature.com/articles/s41598-025-19873-z]
- 76. Exploring the solubility potential of anti-cancer and ... [https://www.sciencedirect.com/science/article/pii/S2212982025002112]
- 77. Optimisation of Supercritical CO2 Extraction from Black ... [https://www.mdpi.com/2076-3417/15/16/9222]
- 78. Optimization of combined subcritical water and CO2 ... [https://pubmed.ncbi.nlm.nih.gov/40089521/]
- 79. Impact of extraction techniques on phytochemical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12343529/]
- 80. Supercritical CO2 Extraction vs. Hexane Extraction and Cold ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11644741/]
- 81. Supercritical carbon dioxide extraction of lipids and ... [https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-025-02632-7]
- 82. Supercritical CO2 extraction of lipids and bioactives from ... [https://link.springer.com/article/10.1007/s10811-025-03534-9]
- 83. Supercritical CO2 and Conventional Extraction of Bioactive ... [https://www.mdpi.com/2223-7747/13/20/2931]
- 84. Application Notes [https://www.supercriticalfluids.com/technical-resources/applications-notes/]
- 85. Supercritical COâ‚‚ extraction of Clinacanthus nutans [https://pmc.ncbi.nlm.nih.gov/articles/PMC12610283/]
- 86. Comparative Analysis of Seed Oils from Six Plant Species [https://pubmed.ncbi.nlm.nih.gov/39683202/]
- 87. A sustainable approach to extracting baobab oil: neat ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra02490k]
- 88. Effects of Extraction Strategies on Yield, Physicochemical ... [https://www.mdpi.com/2304-8158/12/18/3351]
- 89. Supercritical CO2 Extraction Machines Market Trends 2025 ... [https://www.linkedin.com/pulse/supercritical-co2-extraction-machines-market-trends-azc5e/]
- 90. Supercritical Fluid Extraction System Market Size 2025-2032 [https://www.360iresearch.com/library/intelligence/supercritical-fluid-extraction-system]
- 91. Pilot-Scale Optimization of Supercritical CO2 Extraction ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9000775/]
- 92. Simulation and Optimization: A New Direction in Supercritical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10741229/]
- 93. Development and Optimization of Supercritical Fluid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8227983/]
- 94. Supercritical CO2 elimination of solvent residues from ... [https://www.sciencedirect.com/science/article/abs/pii/S0896844621001650]
- 95. Supercritical Fluid Extraction: A Green and Sustainable ... [https://www.ajgreenchem.com/article_221328.html]
- 96. Supercritical Carbon Dioxide Extraction of Four Medicinal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8468049/]
- 97. Supercritical Carbon Dioxide Extraction Equipment Sales ... [https://dataintelo.com/report/global-supercritical-carbon-dioxide-extraction-equipment-sales-market]
- 98. Supercritical CO2 Extraction Market Size & Share [2033] [https://www.globalgrowthinsights.com/market-reports/supercritical-co2-extraction-machines-market-115192]
- 99. United States Supercritical Co2 Extractor Market Size 2025 ... [https://www.linkedin.com/pulse/united-states-supercritical-co2-extractor-gjsue/]
- 100. Designing greener active pharmaceutical ingredients [https://www.sciencedirect.com/science/article/pii/S0928098723002440]